Abstract
Objective
This study was performed to investigate serum prolidase enzyme activity and oxidative stress in patients diagnosed with fibromyalgia (FM).
Methods
The study population consisted of 40 patients with a previous diagnosis of FM and 30 healthy subjects. We measured serum prolidase enzyme activity, total antioxidant status (TAS), total oxidative status (TOS), oxidative stress index (OSI), and paraoxonase-1 (PON-1) levels.
Results
On average, FM patients were diagnosed within 3.2 years of symptom onset, and patients had a mean of 14 tender points. There were no significant differences between patients and controls in age, body mass index, serum TAS, or PON-1 levels. However, patients with FM demonstrated higher serum prolidase activity, TOS, and OSI than the control group. Serum prolidase activity was positively correlated with serum TOS, OSI, and visual analog scale pain and fatigue scores. No correlation was found between serum prolidase activity and FM duration or the average number of tender points.
Discussion
Our results demonstrate a previously unreported association between serum prolidase enzyme activity and FM. Increased prolidase activity may contribute to the pathogenesis of FM, and measuring serum prolidase enzyme activity may be a useful FM biomarker.
Keywords:
Introduction
Fibromyalgia (FM) is a chronic musculoskeletal syndrome characterized by diffuse pain, fatigue, stiffness, and tenderness at specific anatomic sites termed ‘tender points’.Citation1,Citation2 FM is more commonly diagnosed in women than in men, and its etiology remains unknown.Citation1,Citation3 Alterations in collagen metabolism may contribute to FM pathogenesis. Nerve endings located in tender points have a distinctive histological appearance involving organized collagen matrix layers, which may indicate abnormal collagen metabolism at these sites. Specifically, trapezius biopsies from FM patients exhibit a characteristic collagen cuff that surrounds axons, which has been associated with myofibril disorganization contributing to post-exercise pain and stiffness.Citation4 The authors of a previous study suggested that understanding collagen metabolism in FM may be central to understanding its etiology.Citation5
Prolidase is an enzyme that participates in collagen metabolism and is expressed in both human and animal tissues. It participates in the final, rate-limiting step of collagen catabolism.Citation6 Prolidase activity increases when extracellular matrix remodeling occurs, which necessitates a high rate of collagen turnover.Citation7 In a culture system, increased prolidase activity was triggered by increased fibroblast density.Citation8 Altered prolidase activity is also observed in the settings of oxidative stress and diseases characterized by fibrosis.Citation9
Oxidation is a chemical reaction that transfers electrons or hydrogen atoms from a substance to an oxidizing agent, and this can cause cell damage or death. Antioxidants terminate this reaction by removing free radicals and inhibiting various oxidation reactions. When antioxidant levels are reduced, oxidative stress can result in cell structural damage and impaired cell function.Citation10 Oxidative stress has been implicated in FM pathophysiology.Citation1,Citation11,Citation12 Plasma lipid peroxidation (LP) and protein carbonylation both generate reactive oxygen species (ROS) and are increased in patients with FM. Oxidative stress can cause endothelial dysfunction, fibroblast proliferation, and increased type I collagen synthesis.Citation13 In addition, ROS can induce secretion of inflammatory and fibrogenic cytokines.Citation14 There is evidence that FM patients have alterations in muscle metabolism and structure that are reflective of oxygenation abnormalities and/or heightened oxidative stress. Muscle and neural tissue are especially sensitive to free radical damage,Citation15 and aberrant oxygenation has been reported in trigger point areas, which could induce dysregulated pain processing by the central nervous system.Citation9 There are strong relationships among oxidative stress, major depression,Citation16 pain,Citation1,Citation11,Citation17 and fatigue,Citation16 which is commonly observed in FM patients.
Although the contribution of oxidative stress to FM pathophysiology has been assessed in several investigations, the role of serum prolidase in patients with FM has not been studied. The purpose of this study was to investigate serum prolidase enzyme activity and oxidative stress in patients with FM.
Materials and methods
This study was conducted between February and May 2012 by the Physical Therapy and Rehabilitation Department at Dicle University in Turkey. We enrolled a total of 40 patients diagnosed with primary FM, according to the American College of Rheumatology criteria, who were between 18 and 50 years of age, had signed the informed consent form, and did not satisfy any of the exclusion criteria.Citation18 Healthy hospital staff members (n = 30) served as control subjects and were frequency-matched for age and body mass index (BMI). Healthy controls had no signs or symptoms of FM. The entire study population (n = 70) was female. This study was approved by the ethics committee (Dicle University).
Exclusion criteria
Patients were excluded for the following reasons: inflammatory rheumatic disease or infectious or endocrine-related arthropathy; pregnancy or lactation; chronic disorders including coronary artery disease, diabetes mellitus, hypertension, dyslipidemia, or renal disease; malignancy; systemic or local infection in the previous 4 weeks; past or present psychiatric, neurological, autoimmune, or allergy-related disease; or regular use of analgesics, glucocorticoids, immunosuppressive agents, anti-depressants, anti-epileptic drugs, or medication use within 4 weeks of the beginning of the study. None of the patients or control participants had taken drugs or vitamin/nutritional supplements in the 4-week period before blood sample collection. All patients and controls were non-smokers and reported that they consumed a standard diet.
Routine blood tests, erythrocyte sedimentation rate (ESR), total antioxidant status (TAS), total oxidative status (TOS), oxidative stress index (OSI), paraoxonase-1 (PON-1), and prolidase activity were evaluated for all subjects. Demographic and clinical data were obtained from subject interviews, chart reviews, physical examinations, and questionnaires.
FM patients completed the Fibromyalgia Impact Questionnaire (FIQ)Citation19 and Modified Fatigue Impact Scale (M-FIS).Citation20 The clinical severity or the current health status of FM patients was evaluated using the FIQ, which is a self-reported instrument comprising 10 items (physical impairment, feel good, work missed, interference with job, pain, fatigue, morning tiredness, stiffness, anxiety, and depression). Each item was standardized on a scale ranging from 0 to 10. The impact of fatigue was assessed using the M-FIS, which consists of 21 items including 10 related to mental fatigue and 11 related to physical and social fatigue.
All study subjects completed two visual analog scales (VAS) for pain and fatigue; these were scored on a 10-point scale, where 0 represents symptom absence and 10 represents the maximum impact.Citation21 About 4 kg of force was applied via palpation to assess tender points.
Biochemical analyses
After collection, fasting blood samples were immediately centrifuged at 4000 rpm for 10 minutes. Serum samples were then transferred to Eppendorf tubes for storage at −50°C before analysis was performed.
Measurement of the TAS
TAS of serum was determined using a novel automated measurement method, developed by Erel.Citation22 This method produces hydroxyl radicals, which are the most potent biological radical. The sequentially produced radicals, such as brown-colored dianisidinyl radical cations produced by the hydroxyl radical, are also potent. This method measures the antioxidant effect of the sample against the potent free radical reactions, which is initiated by the produced hydroxyl radical. The assay has excellent precision values of lower than 3%. The results are expressed as μmol Trolox equivalent (equiv.)/l.
TOS measurement
Serum TOS was determined using a novel automated measurement method developed by Erel.Citation23 Oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion forms a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide, and the results are expressed in μmol H2O2 equiv./l).
OSI determination
The percent ratio of TOS level to TAS level was considered as the OSI, which indicates the degree of oxidative stress.Citation24,Citation25 OSI was calculated according to the following formula: OSI (arbitrary unit) = TOS (μmol H2O2 equiv./l)/TAS (μmol Trolox equiv./l.
Serum prolidase levels (U/l) were determined using a spectrophotometric method that measures the levels of proline produced by prolidase. The supernatant was diluted two-fold with physiologic saline (0.9% NaCl). Next, 25 µl of the mixture was preincubated with 75 µl preincubation solution (50 mmol/l Tris–HCl buffer (pH 7.0) containing 1 mmol/l glutathione and 50 mmol/l MnCl2) at 37°C for 30 minutes. A total of 100 µl reaction mixture, which was composed of 144 mmol/l gly–pro (pH 7.8), was incubated with 100 µl preincubated sample at 37°C for 5 minutes. To stop the incubation reaction, 1 ml glacial acetic acid was added. After adding 300 µl Tris–HCl buffer (pH 7.8) and 1 ml ninhydrin solution (3 g/dl ninhydrin was dissolved in 0.5 mol/l orthophosphoric acid), the mixture was incubated at 90°C for 20 minutes and then cooled with ice. Absorbance was then measured at a 515-nm wavelength to determine proline content according to the method proposed by Myara et al.,Citation26 which is a modification of Chinard's method.Citation27 The intra- and interassay coefficients of variation were both lower than 7%.
Serum PON-1 levels (U/l) were measured with an Anthos Zenyth 200rt spectrophotometer (Biochrom Ltd., Cambridge, UK) via the modified Eckerson method. Initial paraoxon (diethyl 4-nitrophenyl phosphate) phosphate hydrolysis rates were determined by measuring liberated p-nitrophenol at 405 nm at 37°C (Sigma, St. Louis, MO, USA).Citation28
Statistical analyses
All data were expressed as the mean ± one standard deviation. Kolmogorov–Smirnov tests were performed to determine whether the data were normally distributed. Intergroup comparisons were performed using Mann–Whitney U tests and Student's t-tests. Relationships between variables were analyzed by either Pearson or Spearman correlation analyses depending on how the variables were distributed. Statistical significance was achieved if the P value was less than 0.05. All data were analyzed with SPSS statistical software package 18.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
Demographic and clinical data, as well as blood laboratory results, are displayed in . The mean delay from symptom onset to FM diagnosis was 3.2 years, and the average number of tender points in FM patients was 14. The mean ages were 36.20 ± 7.97 years and 34.30 ± 6.19 years for FM patients and controls, respectively. No statistically significant difference was demonstrated between groups in terms of age (P = 0.266), BMI (P = 0.062), mean hematocrit level (P = 0.253), mean C-reactive protein (CRP) level (P = 0.486), mean ESR (P = 0.848), and mean white blood cell counts (WBC) (P = 0.191). However, the VAS pain and fatigue scores were significantly higher in patients with FM compared with the control group (P = 0.000).
Table 1. Demographics and clinical and laboratory results for FM patients and controls
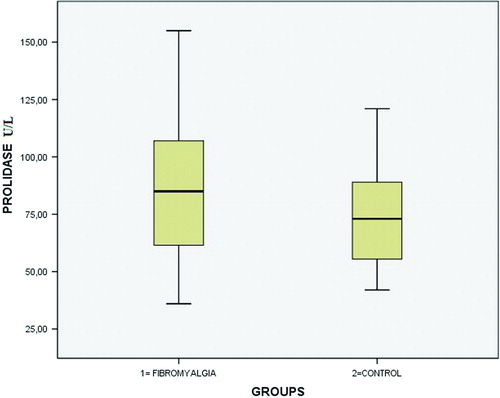
Serum prolidase activity (P = 0.004), TOS (P = 0.006), and OSI (P = 0.001) were all significantly increased in the FM group. Serum TAS levels were lower in FM patients, but this difference was not statistically significant compared with the control group (P = 0.069). Serum PON-1 levels were higher in the FM group, but this was not statistically significant (P = 0.451). Serum prolidase activities are shown in . TAS, TOS, OSI, and PON-1 levels are shown in .
Table 2. Serum TAS, TOS, OSI, and PON-1 levels in control and FM groups
Serum prolidase activity was positively correlated with TOS (P = 0.010, r = 0.305), OSI (P = 0.002, r = 0.410), VAS pain scores (P = 0.010, r = 0.300), and VAS fatigue scores (P = 0.020, r = 0.320). However, serum prolidase activity was negatively correlated with TAS (P = 0.003, r = 0.348). There was no correlation between serum prolidase activity and the average number of tender points (P = 0.618, r = 0.084) or between FM duration and prolidase activity (P = 0.086, r = 0.384). Notably, prolidase and all oxidative stress markers (TAS, TOS, OSI) were correlated with VAS fatigue score. These correlations are summarized in .
Table 3. Correlations between prolidase, TAS, TOS, OSI, PON-1, and clinical variables in control and FM groups
Discussion
Prolidase is a ubiquitous enzyme that catalyzes the rate-limiting step of collagen catabolism and functions in dietary protein degradation.Citation4 Prolidase promotes collagen metabolism because it recycles proline for use in collagen biosynthesis, and this enzyme is dysfunctional in certain pathological conditions.Citation29–Citation32 For instance, free radicals promote oxidative stress and alter prolidase activity, which prevents collagen fibril formation.Citation33 Duong et al.Citation34 observed a statistically significant difference in prolidase activity in keloid tissue compared with normal skin. Therefore, prolidase might be a useful marker in measuring severity of conditions characterized by fibrosis. Moreover, the extent of prolidase activity might have a role in FM pathogenesis; tissue biopsies of tender points exhibit collagen cuffs. Urinary hydroxyproline concentrations are significantly lower in FM patients compared with age- and sex-matched controls, indicating a systemic alteration in collagen metabolism.Citation5 However, there is no literature describing serum prolidase activity in patients with FM. In this study, we observed significantly higher serum prolidase activity in FM patients compared to healthy controls. Additionally, serum prolidase activity was positively correlated with VAS pain and fatigue scores. However, there was no correlation between serum prolidase activity and the average number of tender points or FM duration.
Prolidase is a matrix metalloproteinase, and its activity is affected by oxidative stress.Citation35 Plasma prolidase activities are elevated in chronic tissue inflammation and positively correlate with TAS.Citation36 In this study, the serum prolidase activity was positively correlated with TOS and negatively correlated with TAS. However, we did not observe a relationship between serum prolidase activity and acute phase reactants.
Aberrant pain-regulating mechanisms at the central and peripheral nervous system levels contribute to FM pathophysiology.Citation37 As high oxidant levels were observed in low antioxidant settings in FM patients in this study, it is possible that oxidative stress might contribute to neuropathic pain in FM. OSI is the ratio of TOS to TAS and has been used as a novel indicator of oxidative stress extent.Citation38 A recently published study reported that plasma TAS in FM patients was significantly lower in healthy control subjects. Similarly, another group found that OSI values for FM patients were significantly higher than those for controls.Citation9 Neyal et al.Citation18 also described increased TOS and OSI in patients with FM. We found that in general, serum TAS was lower in FM patients, but this finding was not statistically significant. However, we observed significantly higher serum TOS and OSI levels in FM patients.
Located on high-density lipoprotein (HDL), the PON-1 enzyme hydrolyzes oxidized low-density lipoprotein (LDL). PON-1 also protects HDL from oxidation and is therefore protective against oxidative stress.Citation39 The antioxidant properties of PON-1 then facilitate HDL-mediated protection against atherosclerosis.Citation40,Citation41 Altındag et al.Citation42 reported that patients with FM had significantly lower PON-1 and arylesterase activities than healthy controls. We found that serum PON-1 levels were higher in the FM group compared with the control group; however, this difference was not statistically significant.
Acute phase reactants, such as CRP, ESR, and WBC, are important markers that may aid in making diagnoses and determining medical treatment efficacy for patients with inflammatory diseases. Cordero et al.Citation43 reported that inflammation plays a role in FM pathophysiology in some patients and concluded that oxidative stress and mitochondria are contributing factors for the development of inflammation. Another study found that pro-inflammatory cytokine levels were elevated in serum and biopsies from FM patients.Citation44 Another group described a positive correlation between plasma LP and clinical symptoms for VAS for total FIQ score in FM patients. In this study, we found that levels of acute phase reactants were not significantly different between controls and FM patients. Cordero et al.Citation45 reported a significant correlation between LP in blood mononuclear cells and clinical parameters in FM patients. They also described a positive correlation between plasma LP and clinical symptoms of FM. Here, we found that all oxidative stress markers assessed in plasma (TAS, TOS, and OSI) were correlated with VAS fatigue scores in FM patients.
Our study is a preliminary investigation designed to provide information about serum prolidase activity in patients with FM, and it has several limitations. We assessed a limited sample, and the study was cross-sectional in design. Also, it was not randomized. Assessing prolidase activity in terms of clinical findings would be more informative; however, a subgroup classification could not be done in these small study groups. Further prospective and randomized studies with larger samples are needed to clarify this issue.
Conclusion
Serum prolidase enzyme activity and oxidative stress were both significantly higher in patients with FM compared with healthy controls. These data demonstrate a previously unreported association between serum prolidase enzyme activity and FM. Increased prolidase activity may contribute to FM pathogenesis; however, prospective, randomized control trials are needed to elucidate this possible association and identify prolidase activity levels for diagnosing and treating patients with FM.
References
- Ozgocmen S, Ozyurt H, Sogut S, Akyol O. Current concepts in the pathophysiology of fibromyalgia: the potential role of oxidative stress and nitric oxide. Rheumatol Int 2006;26(7):585–97.
- Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol Suppl 2005;75:6–21.
- Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, et al. Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum 2010;39(6):448–53.
- Gronemann ST, Ribel-Madsen S, Bartels EM, Danneskiold-Samsoe B, Bliddal H. Collagen and muscle pathology in fibromyalgia patients. Rheumatology (Oxford) 2004;43(1):27–31.
- Sprott H, Muller A, Heine H. Collagen crosslinks in fibromyalgia. Arthritis Rheum 1997;40(8):1450–4.
- Surazynski A, Miltyk W, Palka J, Phang JM. Prolidase-dependent regulation of collagen biosynthesis. Amino Acids 2008;35(4):731–8.
- Palka JA, Phang JM. Prolidase activity in fibroblasts is regulated by interaction of extracellular matrix with cell surface integrin receptors. J Cell Biochem 1997;67(2):166–75.
- Myara I, Wolfrom C, Charpentier C, Gautier M, Lemonnier A. Relationship between cell density and prolidase activity in human skin fibroblasts: effects of ascorbate and fructose. Biochimie 1984;66(6):445–9.
- Altindag O, Celik H. Total antioxidant capacity and the severity of the pain in patients with fibromyalgia. Redox Rep 2006;11(3):131–5.
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997;82(2):291–5.
- Cordero MD. Oxidative stress in fibromyalgia: pathophysiology and clinical implications. Reumatol Clin 2011;7(5):281–3.
- Miyamae T, Seki M, Naga T, Uchino S, Asazuma H, Yoshida T, et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep 2013;18(1):12–19.
- Tsou PS, Talia NN, Pinney AJ, Kendzicky A, Piera-Velazquez S, Jimenez SA, et al. Effect of oxidative stress on protein tyrosine phosphatase 1B in scleroderma dermal fibroblasts. Arthritis Rheum 2012;64(6):1978–89.
- Yamamoto T. Autoimmune mechanisms of scleroderma and a role of oxidative stress. Self Nonself 2011;2(1):4–10.
- Akkus S, Naziroglu M, Eris S, Yalman K, Yilmaz N, Yener M. Levels of lipid peroxidation, nitric oxide, and antioxidant vitamins in plasma of patients with fibromyalgia. Cell Biochem Funct 2009;27(4):181–5.
- Cordero MD, de Miguel M, Moreno-Fernandez AM. Mitochondrial dysfunction in fibromyalgia and its implication in the pathogenesis of disease. Med Clin (Barc) 2011;136(6):252–6.
- Inanici F, Yunus MB. History of fibromyalgia: past to present. Curr Pain Headache Rep 2004;8(5):369–78.
- Neyal M, Yimenicioglu F, Aydeniz A, Taskin A, Saglam S, Cekmen M, et al. Plasma nitrite levels, total antioxidant status, total oxidant status, and oxidative stress index in patients with tension-type headache and fibromyalgia. Clin Neurol Neurosurg 2013;115(6):736–40.
- Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol 1991;18(5):728–33.
- Armutlu K, Keser I, Korkmaz N, Akbiyik DI, Sumbuloglu V, Guney Z, et al. Psychometric study of Turkish version of Fatigue Impact Scale in multiple sclerosis patients. J Neurol Sci 2007;255(1–2):64–8.
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46(10):1121–3.
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004;37(2):112–9.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38(12):1103–11.
- Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis 2005;5:95.
- Aslan M, Sabuncu T, Kocyigit A, Celik H, Selek S. Relationship between total oxidant status and severity of diabetic nephropathy in type 2 diabetic patients. Nutr Metab Cardiovasc Dis 2007;17(10):734–40.
- Myara I, Charpentier C, Lemonnier A. Optimal conditions for prolidase assay by proline colorimetric determination: application to iminodipeptiduria. Clin Chim Acta 1982;125(2):193–205.
- Chinard FP. Photometric estimation of proline and ornithine. J Biol Chem 1952;199(1):91–5.
- Eckerson HW, Romson J, Wyte C, La Du BN. The human serum paraoxonase polymorphism: identification of phenotypes by their response to salts. Am J Hum Genet 1983;35(2):214–27.
- Myara I, Myara A, Mangeot M, Fabre M, Charpentier C, Lemonnier A. Plasma prolidase activity: a possible index of collagen catabolism in chronic liver disease. Clin Chem 1984;30(2):211–15.
- Karna E, Surazynski A, Palka J. Collagen metabolism disturbances are accompanied by an increase in prolidase activity in lung carcinoma planoepitheliale. Int J Exp Pathol 2000;81(5):341–7.
- Wolanska M, Sobolewski K, Drozdzewicz M. Integrins and prolidase activity in uterine leiomyoma during tumor growth. Ginekol Pol 2001;72(3):121–6.
- Wolczynski S, Surazynski A, Swiatecka J, Palka J. Estrogenic and antiestrogenic effects of raloxifene on collagen metabolism in breast cancer MCF-7 cells. Gynecol Endocrinol 2001;15(3):225–33.
- Deberg M, Labasse A, Christgau S, Cloos P, Bang Henriksen D, Chapelle JP, et al. New serum biochemical markers (Coll 2–1 and Coll 2–1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 2005;13(3):258–65.
- Duong HS, Zhang QZ, Le AD, Kelly AP, Kamdar R, Messadi DV. Elevated prolidase activity in keloids: correlation with type I collagen turnover. Br J Dermatol 2006;154:820–8.
- Hilali N, Vural M, Camuzcuoglu H, Camuzcuoglu A, Aksoy N. Increased prolidase activity and oxidative stress in PCOS. Clin Endocrinol (Oxf) 2013;79(1):105–10.
- Gencer M, Aksoy N, Dagli EC, Uzer E, Aksoy S, Selek S, et al. Prolidase activity dysregulation and its correlation with oxidative-antioxidative status in chronic obstructive pulmonary disease. J Clin Lab Anal 2011;25(1):8–13.
- Fitzcharles MA, Ste-Marie PA, Pereira JX. Canadian Fibromyalgia Guidelines C. Fibromyalgia: evolving concepts over the past 2 decades. CMAJ 2013;17(185):E645–51.
- Cakmak A, Soker M, Koc A, Erel O. Paraoxonase and arylesterase activity with oxidative status in children with thalassemia major. J Pediatr Hematol Oncol 2009;31(8):583–7.
- Borba EF, Borges CT, Bonfa E. Lipoprotein profile in limited systemic sclerosis. Rheumatol Int 2005;25(5):379–83.
- Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol 2001;21(4):473–80.
- Malin R, Jarvinen O, Sisto T, Koivula T, Lehtimaki T. Paraoxonase producing PON1 gene M/L55 polymorphism is related to autopsy-verified artery-wall atherosclerosis. Atherosclerosis 2001;157(2):301–7.
- Altindag O, Gur A, Calgan N, Soran N, Celik H, Selek S. Paraoxonase and arylesterase activities in fibromyalgia. Redox Rep 2007;12(3):134–8.
- Cordero MD, Diaz-Parrado E, Carrion AM, Alfonsi S, Sanchez-Alcazar JA, Bullon P, et al. Is inflammation a mitochondrial dysfunction-dependent event in fibromyalgia? Antioxid Redox Signal 2013;18(7):800–7.
- Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology (Oxf) 2001;40(7):743–9.
- Cordero MD, Alcocer-Gomez E, Cano-Garcia FJ, De Miguel M, Carrion AM, Navas P, et al. Clinical symptoms in fibromyalgia are better associated to lipid peroxidation levels in blood mononuclear cells rather than in plasma. PLoS One 2011;6(10):e26915.