Abstract
Objective
Numerous factors, including genetic, neurobiological, neurochemical, and psychological factors, are thought to be involved in the development of anxiety disorders. The latest findings show that the pathophysiology of anxiety disorders might be associated with oxidative stress and lipid peroxidation; however, no studies have so far investigated lipid peroxidation markers in children with anxiety disorders. Serum levels of lipid hydroperoxide (LOOH) are a reliable marker of lipid peroxidation. Paraoxonase and arylesterase are two enzymes that protect against such peroxidation, and might also be diagnostic markers. In this study, we investigated whether there are associations between anxiety disorders and lipid peroxidation markers in children, and assessed the diagnostic performance of these markers.
Methods
The study group consisted of 37 patients (children and adolescents) with anxiety disorders. A control group, matched for age and gender, was composed of 36 healthy subjects. Venous blood samples were collected, and LOOH levels and paraoxonase and arylesterase activity were measured.
Results
LOOH levels were significantly higher in the anxiety disorders group than in the control group. There were no significant differences in paraoxonase or arylesterase activities between the patient and the control groups.
Discussion
Lipid peroxidation or oxidative damage might play a role in the aetiopathogenesis of anxiety disorders. LOOH may be a potential biological marker for anxiety disorders in children.
Introduction
Anxiety disorders are the most common psychiatric condition in children and adolescents, and they lead to the subsequent development of other psychiatric comorbidities in adulthood. The lifetime prevalence of anxiety disorders is estimated to reach approximately 30%.Citation1 Males have a higher prevalence rate of the disorder than females.Citation2
Many children experience various types of fears during childhood, some of which are related to their normal development. These anxieties and fears are considered pathological when they become extreme and persistent.Citation3 Pathological anxiety can be identified as an anticipatory response to internal and external threats. Anxiety disorders at pathological levels can cause disruption to the daily life and social functioning of a person. In addition, anxiety disorders presenting as early onset conditions can be risk factors for the development of depressive and other mood disorders occurring later in life.Citation4,Citation5 Some features of anxiety disorders include cognitive (inattention and distractibility, thought blocks, fear of losing control), affective (worry, tension, irritability, despair), behavioural (escape, avoidance, behavioural inhibition), and physical (palpitation, shortness of breath, chest pain, abdominal pain, nausea, vomiting, sweating, chills) symptoms. There are many types of anxiety disorders, including separation anxiety disorder, generalized anxiety disorder (GAD), obsessive compulsive disorder (OCD), panic disorder (PD), post-traumatic stress disorder (PTSD), social anxiety disorder (SAD), and specific phobias.Citation6
Anxiety disorders are complex conditions, with varied aetiologies. A number of factors, including genetic, neurobiological, neurochemical, psychological, and behavioural factors are thought to be involved in the development of anxiety disorders.Citation7 There has been an increase in the number of biological studies of anxiety disorders over the past decade, whereas previously, the psychological, behavioural, and cognitive factors related to anxiety disorders had been the focus of the study. These biological studies have indicated that oxidative damage and lipid peroxidation have a pathophysiological role in anxiety disorders in adult patients, including in PD, OCD, SAD, and GAD.Citation8–Citation11 However, to the best of our knowledge, the possible relation between lipid peroxidation and anxiety disorders has not yet been studied in children and adolescents with anxiety disorders.
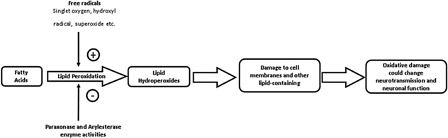
Lipid peroxidation is a well-known example of oxidative damage to cell membranes, lipoproteins, and other lipid-containing structures. Unsaturated phospholipids, glycolipids, and cholesterol can develop as a result of reactions triggered by free radicals (such as hydroxyl radical, singlet oxygen, and superoxide). Lipid hydroperoxide (LOOH) is the primary product of the oxidation of polyunsaturated fatty acids, and it can be used as a biomarker to detect and quantify early stage lipid peroxidation.Citation12 The enzymes, paraoxonase and arylesterase, circulate in the bloodstream, and are associated with high-density lipoprotein. These two enzymes protect lipids from peroxidation, and thus, they exhibit antioxidant features. In the brain, oxidative damage can change neurotransmission, neuronal function, and even brain activity,Citation13,Citation14 and this condition might predispose the individual to neurodegenerative and neuropsychiatric diseases. The relationships between paraoxonase and arylesterase enzyme activity, oxidative damage, and lipid peroxidation are shown in .Citation11 Lipid peroxidation has been linked to a variety of neuropsychiatric disorders, including depression, schizophrenia, bipolar mood disorders, attention deficit hyperactivity disorder, and Alzheimer's disease.Citation15–Citation18
In this study, we aimed to investigate whether there are any associations between anxiety disorders and the activity of paraoxonase and arylesterase (which is associated with antioxidative metabolism) or levels of LOOH, an important marker of lipid peroxidation. In addition, we analysed whether these biological markers could be used as diagnostic tools for anxiety disorders.
Methods
Patients and controls
The study included 37 patients with anxiety disorders and 36 healthy volunteer controls from the Child and Adolescent Psychiatry Clinic at the Ankara Pediatric Hematology Oncology Training and Research Hospital. All of the patients were screened for psychiatric disorders using the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (K-SADS-PL), which is based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria.Citation19 We used the Turkish adaptation of this form, created by Gökler et al.Citation20 Patients with a comorbid psychiatric, neurological, or genetic disorder were excluded along with patients with a history of chronic systemic diseases, such as endocrinal and allergic diseases. Patients who had previously used psychotropic drugs were excluded as well. The control group consisted of healthy children who were matched for age and gender with the anxiety disorders group. Parents of the subjects were given a complete description of the study, and all provided written informed consent. The study was approved by the ethics committee of the Ankara Pediatric Hematology Oncology Training and Research Hospital.
Blood sampling
Venous blood samples were collected from an antecubital vein of each patient at 09.00 hours after a 12-hour overnight fast, and a 10 ml sample of venous blood was placed into a biochemistry tube. The biochemistry tubes were centrifuged at 2000 g for 15 minutes after an incubation period of 30 minutes. The remaining serum specimens were kept at −20°C until analyses were performed.
Measurement of LOOH levels
Serum LOOH levels were measured with a ferrous ion oxidation–xylenol orange (FOX-2) assay, which involves the oxidation of ferrous ion to ferric ion by various oxidants. The ferric ion is then measured with xylenol orange. The LOOH levels are reduced by the application of triphenyl phosphine (TPP), which is a specific reductant for lipids. LOOH levels can be estimated as the difference in values in the absence or presence of TPP. Citation21
Measurement of paraoxonase and arylesterase activity
Paraoxonase and arylesterase activity was measured using paraoxon and phenylacetate substrates.
The rate of paraoxon hydrolysis (diethyl-p-nitrophenyl phosphate) was measured by monitoring the increase of absorbance at 412 nm at 37°C. The amount of generated p-nitrophenol was calculated from the molar absorptivity coefficient at pH 8, which was 17 000 mol/cm, and paraoxonase activity was expressed as U/l serum.
Phenylacetate was used as a substrate to measure arylesterase activity. Enzyme activity was calculated from the molar absorptivity coefficient of the produced phenol, 1310 mol/cm, with 1 U of arylesterase activity defined as 1 µmol phenol generated per minute under the above conditions, and expressed as U/l serum.Citation22,Citation23
Statistical analysis
SPSS® (version 11.5 for Windows; IBM/SPSS, Chicago, IL, USA) was used to analyse the data statistically. With normally distributed and homogeneous variables, the significant differences between the groups were estimated using two-tailed t-tests. Bivariate comparisons were examined via Spearman's correlation coefficients, with values corrected for ties. In addition, χ2 tests were used to evaluate categorical data. Differences were considered significant at P < 0.05.
Results
In the anxiety disorders group (n = 37), the age of the participants was 11.2 ± 3.1 years (mean ± SD; range 6–16 years), while that of the control group was 11.1 ± 2.6 years (range 6–16 years). In the anxiety disorders group, 14 of the patients were male and 23 were female; in the control group, 17 of the participants were male and 19 were female. There was no difference in mean age (t = 0.193, P = 0.84) or gender (χ2 = 0.658, P = 0.41) distribution between the groups. The demographic data and subtypes of anxiety disorders are summarized in .
Table 1. Demographic data and subtypes of anxiety of the study participants
LOOH levels were significantly higher in the anxiety disorders group than in the control group (P = 0.015, t = 2.5). There were no significant differences in paraoxonase (P = 0.427, t = 0.8) or arylesterase (P = 0.612, t = 0.5) activity between the patient and the control groups ().
Table 2. Serum levels of parameters in study subjects
Age was not correlated with LOOH levels or with paraoxonase and arylesterase activity in patients with anxiety disorders, and there were no significant differences between male and female patients (P > 0.05).
Discussion
This is the first study to investigate serum lipid peroxidation markers in children with anxiety disorders. We investigated the serum LOOH levels in child and adolescent patients with anxiety disorders, and found that serum LOOH levels were significantly higher in patients with anxiety disorders than in the normal healthy control subjects. The most studied oxidant marker in adult patients with anxiety disorders is malondialdehyde (MDA). LOOH and MDA are the products of the oxidation of polyunsaturated fatty acids. Many research groups have reported elevated MDA levels in patients with OCD, PD, or SAD.Citation24,Citation25,Citation10 Similarly, another research study reported elevated LOOH levels in patients with GAD.Citation11
Lipid peroxidation is a degenerative process that affects cell membranes and other lipid-containing structures under conditions of oxidative stress.Citation12,Citation13 Oxidative stress is a biological condition that is characterized by the production of excessive amounts of oxidants, decreased levels of antioxidants, or both.Citation26 The human brain, as the most metabolically active tissue in the body, produces a comparatively large amount of free radicals. In addition, the brain is rich in the precise fatty acid composition needed for oxidation, and has relatively modest antioxidant defences.Citation27 Consequently, the brain is highly vulnerable to oxidative stress,Citation27 and this condition might predispose certain individuals to neurodegenerative and neuropsychiatric diseases. Structural imaging studies have indicated bilaterally decreased hippocampus volumes in anxiety disorders.Citation28,Citation29 We suggest that oxidative metabolism may contribute to the formation of anxiety disorders by affecting neuronal structure.
The other finding of this study was that there were no significant differences in paraoxonase and arylesterase activity between the patient and the control groups. The relation between anxiety disorders and the activity levels of a great number of antioxidant enzymes has previously been studied. In a recent study, paraoxonase enzyme activity was found to be lower in adults with GAD, although no significant difference in arylesterase activity was found.Citation11 So far, the most studied antioxidant enzymes in psychiatric disorders are superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT). In one study, SOD, GSH-Px, and CAT enzyme levels were found to be significantly higher in patients with SAD.Citation10 In a different study, higher SOD and GSH-Px activity levels were reported in patients with PD.Citation25 However, a third study, on PTSD, did not find any significant changes in SOD, GSH-Px, or CAT activity.Citation30
The restlessness, irritability, over-excitement, and worrying present in anxiety disorders can cause stress in daily living. Stress challenges an organism's homoeostatic mechanisms.Citation31 Recent studies have shown a relation between excessive production of toxic oxygen metabolites and psychological stress. In the human and animal studies, psychological stress was found to be related to induction of oxidative damage.Citation32–Citation34 Aschbacher et al.Citation34 reported recently that exposure to chronic stress promotes oxidative damage, causing ceaseless activation of the hypothalamic–pituitary–adrenal axis. In a study in animals, high anxiety levels significantly increased oxidative stress.Citation32 Similarly, it has been shown that oxidative stress could trigger anxiety-related behaviour in mice.Citation35 In addition, oxidative stress has been shown to decline with treatment in cases of anxiety disorder.Citation8,Citation36 All of these results suggest that oxidative stress can play a role in the aetiopathogenesis of anxiety disorders.
In the present study, there was no significant correlation between measured LOOH levels and antioxidant enzymes and patient demographics, such as gender and age. These results suggest that increased lipid peroxidation in children with anxiety disorders might be related to the disorder itself.
Until recently, there has been no method of determining any specific ‘diagnostic marker’ for anxiety disorders. Bulut et al.Citation11 reported recently that ‘products of lipid oxidation’ (which would include LOOH), might be used as a diagnostic factor for GAD. It has long been known that lipid peroxidation reactions are induced by oxidative stress.Citation37 In addition, oxidative damage has been shown to be related to the psychopathology of anxiety disorders.Citation10,Citation24,Citation25 Our results also suggest that increased serum LOOH levels might have diagnostic value for children and adolescents with anxiety disorders.
The most prominent limitation of this study is the relatively small sample size, which may not represent all types of anxiety disorders. In addition, owing to the heterogeneity of the anxiety diagnosis in the participants, we could not evaluate the correlation between disease severity and serum lipid peroxidation markers. However, exclusion of any comorbid psychiatric disorders in the anxiety samples increased the reliability of our study.
Lipid peroxidation in the neurons could induce brain inflammation. Animal studies have shown that oxidative stress and inflammation in the brain, adrenal glands, and systemic circulation may play a critical role in the development of anxiety disorders such as PTSD.Citation38 In future studies, simultaneous measurement of lipid peroxidation and inflammation would be beneficial in assessing the cumulative effect of lipid peroxidation in the aetiology of anxiety disorders. In the present study, lipid peroxidation markers were assessed in patients who were not receiving treatment for anxiety disorders. Therefore, there is a need for further evaluation of changes in serum lipid peroxidation markers in the treatment of children with anxiety disorders.
Conclusion
Remarkably high levels of LOOH, along with no statistical changes in paraoxonase and arylesterase activity, suggest an oxidative imbalance in paediatric patients with anxiety disorders. Therefore, lipid peroxidation or oxidative damage might have a role in the aetiopathogenesis of anxiety disorders, and LOOH might be a potential biological marker for anxiety disorders in children. Studies with larger samples are necessary to increase our knowledge about the role of lipid peroxidation in children with anxiety disorders.
Acknowledgement
We thank the children who participated as volunteers in this study, as well as their families.
References
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62(6):593–602.
- Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med 1998;28(1):109–26.
- Weinberger DR. Anxiety at the frontier of molecular medicine. N Engl J Med 2001;344(16):1247–9.
- Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry 2007;64(8):903–12.
- Duffy A, Horrocks J, Doucette S, Keown-Stoneman C, McCloskey S, Grof P. Childhood anxiety: an early predictor of mood disorders in offspring of bipolar parents. J Affect Disord 2013;150(2):363–9.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text-Revision (DSM-IV-TR). American Psychiatric Association, Washington (DC); 2000.
- Cates M, Wells BG, Thatcher GW, Anxiety disorders. In: Herfindal ET, Gourley DR, (eds.) Textbook of therapeutics drug and disease management. Baltimore: Williams and Wilkins; 1996. p. 1073–93.
- Herken H, Akyol O, Yilmaz HR, Tutkun H, Savas HA, Ozen ME, et al. Nitric oxide, adenosine deaminase, xanthine oxidase and superoxide dismutase in patients with panic disorder: alterations by antidepressant treatment. Hum Psychopharmacol 2006;21(1):53–9.
- Selek S, Herken H, Bulut M, Ceylan MF, Celik H, Savas HA, et al. Oxidative imbalance in obsessive compulsive disorder patients: a total evaluation of oxidant-antioxidant status. Prog Neuropsychopharmacol Biol Psychiatry 2008;32(2):487–91.
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Antioxidant enzyme and malondialdehyde levels in patients with social phobia. Psychiatry Res 2008;159(1–2):95–100.
- Bulut M, Selek S, Bez Y, Karababa IF, Kaya MC, Gunes M, et al. Reduced PON1 enzymatic activity and increased lipid hydroperoxide levels that point out oxidative stress in generalized anxiety disorder. J Affect Disord 2013;150(3):829–33.
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res 1998;39(8):1529–42.
- Nunez EA. Fatty acids and cell signalling. Prostaglandins Leukot Essent Fatty Acids 1993;48(1):1–4.
- Delion S, Chalon S, Hérault J, Guilloteau D, Besnard JC, Durand G. Chronic dietary g-linolenic acid deficiency alters dopaminergic and serotonergic neurotransmission in rats. J Nutr 1994;124(12):2466–76.
- Bilici M, Efe H, Köroğlu MA, Uydu HA, Bekaroğlu M, Değer O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 2001;64(1):43–51.
- Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct 2002;20(2):171–5.
- Ceylan M, Sener S, Bayraktar AC, Kavutcu M. Oxidative imbalance in child and adolescent patients with attention-deficit/hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry 2010;34(8):1491–4.
- Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer's disease. Redox Rep 2002;7(4):199–206.
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997;36(7):980–8.
- Gokler B, Unal F, Pehlivanturk B, Kultur EÇ, Akdemir D, Taner Y. Schedule for affective disorders and schizophrenia for school-age childrenpresent and lifetime version – the validity and reliability of adaptation in Turkish. Çocuk ve Gençlik Ruh Saglıgı Dergisi 2004;11:109–16.
- Nourooz-Zadeh J. Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol 1999;300:58–62.
- Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/ arylesterase polymorphism. Am J Hum Genet 1983;35(6):1126–38.
- Haagen L, Brock A. A new automated method for phenotyping arylesterase (EC 3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenylacetate. Eur J Clin Chem Clin Biochem 1992;30(7):391–5.
- Behl A, Swami G, Sircar SS, Bhatia MS, Banerjee BD. Relationship of possible stress-related biochemical markers to oxidative/antioxidative status in obsessive-compulsive disorder. Neuropsychobiology 2010;61(4):210–4.
- Kuloglu M, Atmaca M, Tezcan E, Ustundag B, Bulut S. Antioxidant enzyme and malondialdehyde levels in patients with panic disorder. Neuropsychobiology 2002;46(4):186–9.
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008;11(6):851–76.
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem 2006;97(6):1634–58.
- Yang B, Zhou Y, Xia J, Xia L, Wang C. The study of the volume and 1HMRS of the hippocampus in posttraumatic stress disorder. Chin J Radiol 2006;40(1):36–40.
- Chen J, Shi S. A review of neuroimaging studies of anxiety disorders in China. Neuropsychiatr Dis Treat 2011;7:241–9.
- Tezcan E, Atmaca M, Kuloglu M, Ustundag B. Free radicals in patients with post-traumatic stress disorder. Eur Arch Psychiatry Clin Neurosci 2003;253(2):89–91.
- Pego JM, Sousa JC, Almeida OF, Sousa N. Stress and the neuroendocrinology of anxiety disorders. Curr Top Behav Neurosci 2010;2:97–117.
- Rammal H, Bouayed J, Younos C, Soulimani R. The impact of high anxiety level on the oxidative status of mouse peripheral blood lymphocytes, granulocytes and monocytes. Eur J Pharmacol 2008;589(1–3):173–5.
- Li Q, Zhang M, Chen YJ, Wang YJ, Huang F, Liu J. Oxidative damage and HSP70 expression in masseter muscle induced by psychological stress in rats. Physiol Behav 2011;104(3):365–72.
- Aschbacher K, O'Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 2013;38(9):1698–708.
- Rammal H, Bouayed J, Younos C, Soulimani R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun 2008;22(8):1156–9.
- Ersoy MA, Selek S, Celik H, Erel O, Kaya MC, Savas HA, et al. Role of oxidative and antioxidative parameters in etiopathogenesis and prognosis of panic disorder. Int J Neurosci 2008;118(7):1025–37.
- Matsumoto K, Yobimoto K, Huong NT, Abdel-Fattah M, Van Hien T, Watanabe H. Psychological stress-induced enhancement of brain lipid peroxidation via nitric oxide systems and its modulation by anxiolytic and anxiogenic drugs in mice. Brain Res 1999;839(1):74–84.
- Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS ONE 2013;8(10):e76146.