Abstract
Background
The metabolic syndrome (MetS) is a complex of multiple risk factors that contribute to the onset of cardiovascular disorder, including lowered levels of high-density lipoprotein (HDL) and abdominal obesity. Smoking, mood disorders, and oxidative stress are associated with the MetS. Paraoxonase (PON)1 is an antioxidant bound to HDL, that is under genetic control by functional polymorphisms in the PON1 Q192R coding sequence.
Aims and methods
This study aimed to delineate the associations of the MetS with plasma PON1 activity, PON1 Q192R genotypes, smoking, and mood disorders (major depression and bipolar disorder), while adjusting for HDL cholesterol, body mass index, age, gender, and sociodemographic data. We measured plasma PON1 activity and serum HDL cholesterol and determined PON1 Q192R genotypes through functional analysis in 335 subjects, consisting of 97 with and 238 without MetS. The severity of nicotine dependence was measured using the Fagerström Nicotine Dependence Scale.
Results
PON1 Q192R functional genotypes and PON1 Q192R genotypes by smoking interactions were associated with the MetS. The QQ and QR genotypes were protective against MetS while smoking increased metabolic risk in QQ carriers only. There were no significant associations between PON1 Q192R genotypes and smoking by genotype interactions and obesity or overweight, while body mass index significantly increased MetS risk. Smoking and especially severe nicotine dependence are significantly associated with the MetS although these effects were no longer significant after considering the effects of the smoking by PON1 Q192R genotype interaction. The MetS was not associated with mood disorders, major depression or bipolar disorder.
Discussion
PON1 Q192R genotypes and genotypes by smoking interactions are risk factors for the MetS that together with lowered HDL and increased body mass and age contribute to the MetS.
Introduction
The metabolic syndrome (MetS) is a combination of risk factors that are associated with an increased risk for cardiovascular disorder (CVD), cancer, type 2 diabetes mellitus, depression, and all-cause mortality.Citation1–Citation3 There are different case definitions for the MetS (e.g. the criteria of the International Diabetes Foundation, the National Cholesterol Education Program, and the World Health Organization), which all consider similar risk factors, including increased central obesity as measured by means of waist circumference, increased fasting glucose (insulin resistance) and triglyceride levels, lowered high-density lipoprotein (HDL) levels, and increased blood pressure.Citation1,Citation2,Citation4,Citation5 The MetS frequently coexists with obesity, as measured by an increased body mass index (BMI).Citation6 In some studies, both the MetS and BMI contribute to increased CVD risk, whereas in other studies the MetS, but not BMI, predicts CVD risk.Citation6,Citation7
The key components in the pathophysiology of the MetS syndrome are central obesity, insulin resistance and an increased flux of fatty acids stimulating elevated glucose production, and secretion of triglycerides and very low-density lipoproteins (LDLs).Citation2,Citation8 Lowered levels of HDL cholesterol and increased levels of LDL and leptin are accompanying key components of the MetS.Citation4 Other theories, on the other hand, stress that central obesity is a compensatory mechanism and protects other non-adipose, lipid-sensitive tissues from the increased fatty acid spill over due to excess calories and dietary lipids in the presence of increased leptin resistance.Citation9
New pathways that play a role in the MetS are activation of immune-inflammatory and oxidative and nitrosative stress (O&NS) pathways as indicated by increased levels of pro-inflammatory cytokines, acute phase proteins, and biomarkers of increased lipid peroxidation.Citation10 In accordance with the findings on activated O&NS pathways, the MetS is also accompanied by lowered antioxidant defenses, as shown by reduced total antioxidant potential in the plasma.Citation11
Paraoxonase/arylesterase 1 (PON1, EC 3.1.8.1) is an antioxidant enzyme that is bound to plasma HDL and determines part of the antioxidant capacity of HDL.Citation12,Citation13 PON1 associated with HDL attenuates the production of reactive oxygen species (hydrogen peroxide) thereby contributing to lowered production of oxidized LDL.Citation14 These antioxidant and anti-inflammatory effects of PON1 may explain that lowered levels are associated with an increased risk for CVD and insulin resistance.Citation15–Citation17 There is now evidence that the MetS is accompanied by lowered plasma PON1 activity or PON1 concentrations.Citation18–Citation23
PON1 is under genetic control whereby functional polymorphisms at the 192 position Q→R determine the enzymatic activities and consequently increase risk to different diseases.Citation24 For example, the R allele and the RR genotype are associated with an increased risk for CVD, such as ischemic stroke.Citation24 The Q isoform confers lowered protective activity against oxidation of LDL and HDL.Citation25 In a recent study, we found that PON1 Q192R genotypes were associated with an increased Castelli 1 index, suggesting increased atherogenic potential (de Souza-Nogueira et al., submitted). There is one study showing that PON1 QR and RR genotypes significantly increase the risk of MetS.Citation26
Smoking is one of the environmental factors that may increase the risk of MetS. Thus, in a number of smaller studies an association between smoking and the MetS has been described.Citation27 A meta-analysis based on 13 studies and 56.691 subjects and 8.688 cases showed a significant positive relationship between smoking and the MetS.Citation28 Slagter et al.Citation29 in a large-scaled study showed that there was a positive association between smoking and the MetS, independent of BMI class and gender. Smoking not only affects the metabolism of fatty acids but also induces inflammatory reactions, increases lipid peroxidation, and lowers PON1 levels.Citation30,Citation31 In addition, we recently observed that an interaction pattern between smoking and PON1 genotypes predicts an increased Castelli 1 index and therefore could be an important risk factor for the MetS (submitted).
Another factor that is related to an increased risk towards MetS is the presence of both unipolar and bipolar depression.Citation32,Citation33 These mood disorders show a high degree of comorbidity with obesity, CVD, and diabetes type 2.Citation34 While increased rates of central obesity in mood disorders may be one factor explaining the comorbidity with the MetS, other shared processes are lowered HDL cholesterol and activated immune-inflammatory and O&NS pathways, and common environmental risk factors such as diet, smoking, and physical activity.Citation34–Citation40 Moreover, patients with mood disorders have an increased rate of current smokingCitation41, while current smoking, interactions between PON1 Q192R genotypes and smoking, and reduced plasma PON1 levels are also risk factors for unipolar depression or bipolar disorder.Citation42 Possible interactions between smoking, mood disorders, and PON1 Q192R genotypes could therefore play a role in the MetS.
The aim of the present study was to examine whether lowered PON1 activity and PON1 functional Q192R polymorphisms, e.g. the RR genotype, current smoking, and mood disorders or their interactions may increase the odds to MetS, while controlling for other putative intervening factors, such as BMI, age, gender, and sociodemographic data.
Subjects and methods
Subjects
In this study, we included 335 subjects of Caucasian, African, Asian, and mixed ethnicities and aged between 18 and 60 years old. The subjects were recruited from staff at the Londrina State University and an outpatient ambulatory of smoking cessation program, UEL, Parana, Brazil, and comprised 238 subjects with the MetS and 97 subjects without the MetS; 146 subjects with mood disorders, that is unipolar major depression (n = 91) or bipolar disorder (n = 45), versus 199 subjects without mood disorders, and 144 smokers versus 191 non-smokers. We have excluded subjects (a) with abnormal laboratory tests, including aspartate transaminase, alanine transaminase, hemogram, urea, and creatinine; (b) who had been taken immunomodulatory drugs, including glucocorticoids and antiviral medications; and (c) with other major medical illness, e.g. diabetes type 1 and 2, chronic obstructive pulmonary disease, (auto)immune disorders, inflammatory bowel disease, CVD, and neuroinflammatory disorders. Patients with mood disorders were excluded if they had a lifetime history of other axis 1 Diagnostic and Statistical Manual of Mental Disorders (DSM) IV diagnoses, such as organic mental disorders, schizophrenia, substance abuse disorders, anxiety disorders, etc., while subjects without mood disorders were excluded for any axis I DSM-IV diagnosis. The study was approved by the Institutional Review Board of the UEL (number 250/2010) and all subjects gave written informed consent prior to participating in this study.
Methods
The diagnosis of MetS was made using the diagnostic criteria of the International Diabetes Federation, i.e. three of the five criteria should be present: (a) abdominal obesity, that is waist circumference ≥90 cm for men and ≥80 cm for women in South Asian and South Americans and ≥94.0 cm for men and ≥80.0 cm for women in Caucasians; (b) low HDL cholesterol, that is <40 mg/dl in men and <50 mg/dl in women, or on hypolipidemic drugs; (c) hypertriglyceridemia, that is ≥150 mg/dl, or on a hypolipidemic agent; (d) increased fasting glucose, that is ≥100 mg/dl, or on oral antidiabetic medication; (e) increased average blood pressure, that is ≥130/85 mmHg, or currently taking antihypertensive medication.Citation2,Citation5,Citation43 We measured waist circumference during expiration, in a standing and relaxed position, at the midline between the lower costal margins and the iliac crest parallel to the floor. We measured blood pressure using a mercury sphygmomanometer on the right arm and used the mean value of two measurements that were carried out 5 minutes apart. We calculated the BMI as weight (in kg) divided by square of height (in mCitation2).
The diagnosis of mood disorders was made by senior psychiatrists employing a validated Portuguese translation of the semi-structured interview of the DSM-IV.Citation44 We have examined the effects of the two mood disorders combined and or of each mood disorder, either major depression or bipolar disorder separately. We combined patients in the acute phase of illness and patients in partial or total remission. We measured the severity of depression using a validated Portuguese translation of the Hamilton Depression Rating Scale (HAM-D), which was adapted for use in the Brazilian population.Citation45 The diagnosis ‘nicotine dependence’ was made according to DSM-IV criteria. The severity of dependence was estimated using the Fagerström Nicotine Dependence Scale.Citation46 We used the six items of this scale to examine possible relationships between aspects of nicotine dependence with the MetS, while we used the total score as an index for the severity of nicotine dependence. We also divided the subjects into these with severe nicotine dependence (total score ≥5) versus those with moderate dependence (total score <4).Citation46 We used a self-report questionnaire to collect sociodemographic and clinical characteristics, including years of education, marital status (single, stable relationship, separated, or widowed), ethnicity (Caucasian, Asian, African, and mixed), use of alcohol (no use, monthly, weekly, daily use), and use of statins and any other medications.
Biomedical assays
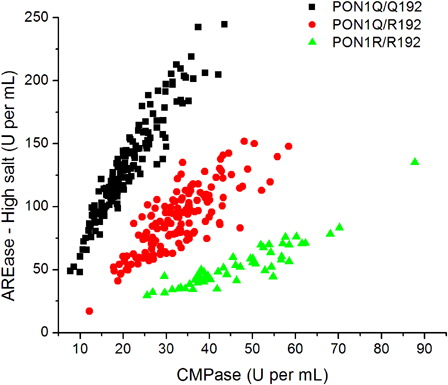
Fasting blood (12–14 hours) was collected in all subjects for the assays of plasma PON1 activity, PON1 Q192R genotypes, and HDL cholesterol. PON1 status, that is PON1 plasma activity and PON1 Q192R functional genotypes or activity phenotypes, were measured as described before.Citation47 In short, the substrates were phenylacetate (PA, Sigma, St Louis, MO, USA) and 4-(chloromethyl)phenylacetate (CMPA, Sigma) and the analysis was conducted in a microplate spectrophotometer reader (EnSpire, Perkin Elmer, NY 14831, USA) using ultraviolet transparent 96-well microplates. All assays were carried out in triplicate and replicates that varied by >10% were repeated. Briefly, CMPA hydrolysis was measured at 280 nm for 4 minutes at 25°C using 20 µl of plasma diluted 1:40 in dilution buffer (20 mmol/l Tris-HCl (pH 8.0), 1.0 mmol/l CaCl2). PA hydrolysis under high salt conditions was measured at 270 nm for 4 minutes at 25°C using 20 µl of plasma diluted 1:40 in dilution buffer. High salt media was composed by PA added to 2 mol/l NaCl, 20 mmol/l Tris-HCl (pH 8.0), 1.0 mmol/l CaCl2. The results obtained with these two assays were used to plot a two-dimensional enzyme activity graphic that displays rates of arylesterase activity (PA hydrolysis) under high salt conditions versus CMPase activity (CMPA hydrolysis). Since the polymorphism Q192R confers differential catalytic activity against these two substrates, the plot splits the population into the three functional position 192 genotypes (QQ, QR, and RR). Measurement of PA hydrolysis at low salt concentrations reveals plasma PON1 total activity, since at this condition PON1 Q192R polymorphism does not influence PON1 catalytic activity against PA.Citation47 For this assay, rates of hydrolysis of PA under low salt conditions were measured at 270 nm for 4 minutes at 25°C using 20 µl of plasma diluted 1:80 in dilution buffer.
shows the division of the participants into the three functional PON1 Q192R polymorphisms: 151 individuals (45.1%) were homozygous for the PON1*192Q allele; 133 (39.7%) were heterozygous; and 51 (15.2%) were homozygous for the PON1*192R allele. Regarding PON1 plasmatic activity, which is determined by the hydrolysis of PA under low salt conditions, it varied from 47.67 to 414.60 U/ml (data not shown).
Figure 1. Functional genotyping for the PON1 Q192R polymorphism through the hydrolysis of CMPA versus PA under high salt condition. Each data point indicates one individual.
HDL cholesterol was assayed using an automated method in a clinical chemistry system, Dimension® RXL (Siemens Healthcare Diagnostics Inc., Newark, DE, USA). This assay measures HDL cholesterol without sample pretreatment or specialized centrifugation steps. HDL cholesterol was used as a surrogate marker of HDL. Triglycerides and glucose were determined using automated methods in a clinical chemistry system, Dimension® RXL (Siemens Healthcare Diagnostics Inc.). The inter-assay coefficients of variability for all analytes were <5.0%.
Statistics
Analysis of variance (ANOVA) was used to check the differences in continuous variables between different categories. The Mann–Whitney U test was employed to examine intergroup differences in the case variables that were not normally distributed. Analyses of contingence tables were checked using χ2 tests to assess differences in the distribution of variables among study groups. We used Pearson's correlation coefficients to assess the relationships between variables. Binary logistic regression analyses were employed to delineate the associations between a dichotomous dependent variable (the presence or absence of a characteristic), i.e. the diagnosis MetS versus no MetS, and a set of independent variables, e.g. PON1 status and smoking and their interactions, and clinical/sociodemographic data. The logistic regression coefficients are employed to estimate odds ratios (B with 95% upper and lower confidence intervals for each explanatory variable in the final model). Multinomial regression analysis was used to investigate the associations between a dependent variable with more than two categories, i.e. three groups divided according to the BMI, and a set of independent variables, e.g. PON1 status and smoking and their interactions, and clinical/sociodemographic data. We used transformations to normalize the distribution of PON1 activity and years of education (ln transformation). We analyzed the data using the SPSS Versions 15 and 19. Statistical significance was set at α = 0.05 (two-tailed).
Results
Demographic data
displays the demographic data of the 335 subjects subdivided into those with and without MetS. We did not use a P-correction to examine the multiple contrasts as these univariate analyses were used a priori to delineate the possible relevant variables to be consecutively employed as determinants of independent association with the MetS or obesity/overweight in ultimate multivariate analyses. There were no significant differences in age and gender between both groups. There was a significant association between smoking and MetS with more smokers in the MetS group. Likewise, there was a significant association between MetS and groups divided according to non-smoking and moderate and severe nicotine dependence. There was no significant association between PON1 Q192R genotypes and MetS. There were similarly no significant differences in PON1 activity between subjects with and without the MetS. Subjects with a MetS had significantly lower levels of HDL cholesterol and higher levels of triglycerides and glucose, waist circumference, systolic blood pressure, and BMI than those without MetS. Likewise, there was a significant association between MetS and groups divided based on BMI values. There were no associations between MetS and mood disorders, unipolar depression and bipolar disorder, HAM-D, education, ethnicity, marital status, and use of statins or alcohol. The use of any medication was significantly greater in subjects without MetS than in those with MetS.
Table 1. Demographic data of the 355 subjects in the study divided into those with (n = 97) and without (n = 238) the MetS
The MetS and the Fagerström Nicotine Dependence Scale in smokers
shows that in smokers there was no significant association between the MetS and moderate versus heavy nicotine dependence (χ2 = 0.00, df = 1, P = 0.949). also shows that in smokers the Fagerström Nicotine Dependence Scale score did not differ significantly between those with and without the MetS. There was a significant relationship between MetS and the fourth item of the Fagerstrom scale, i.e. ‘how many cigarettes per day do you smoke?’, i.e. 10 or less (8/11), 11–20 (16/49), 21–30 (11/20), and 31 or more (17/12) (χ2 = 10.39, df = 3, P = 0.016). There were no significant associations between MetS and any of the other items of the Fagerström Nicotine Dependence Scale.
The MetS, smoking, and PON1 functional genotypes
shows the stratification of the study population on the basis of PON1 Q192R genotypes and smoking while indicating for each subgroup the prevalence of MetS. The prevalence of MetS was significantly different in subgroups formed on the basis of smoking and QQ genotype but not QR and RR genotypes. In non-smokers, there were significantly more individuals with a MetS in QR carriers (χ2 = 4.89, df = 1, P = 0.027) and especially RR carriers (χ2 = 11.3, df = 1, P < 0.001) than in QQ carriers. In smokers, no such differences were observed.
Table 2. Stratification of the study population on the basis of PON1 Q192R genotypes and smoking and indicating for each subgroup the (relative) prevalence of the MetS
shows the results of two bivariate logistic regression analyses with MetS versus no MetS as a dichotomous dependent variable. Entering current smoking as the only explanatory variable showed that smoking was significantly associated with the MetS (Wald = 6.21, df = 1, P = 0.013). Entering moderate and severe nicotine dependence versus non-smoking as explanatory variables showed that severe (Wald = 5.27, df = 1, P = 0.022), but not moderate (Wald = 2.61, df = 1, P = 0.11) nicotine dependence predicted the MetS. Entering smoking 10 or less, 11–20, 21–30, or 31 or more cigarettes per day versus non-smoking as explanatory variables showed that only 31 or more cigarettes per day significantly predicted the MetS (Wald = 13.59, df = 1, P < 0.001).
Table 3. Results of bivariate logistic regression analyses with MetS versus no MetS as dependent variable and the listed variables as explanatory variables
The first logistic regression analysis in shows that PON1 Q192R genotype (QQ is inversely associated with MetS) and the smoking by PON1 Q192R interaction pattern, but not smoking per se, increased the odds of belonging to the MetS group (χ2 = 16.14.42, df = 5, P = 0.006; Nagelkerke = 0.067; the number of correctly classified cases was 71.0%). The interaction showed that smoking by QQ carriers increased the odds to MetS.
The second logistic regression in shows that the effects of PON1 Q192R genotypes (QQ and QR are inversely associated with MetS) and the interaction smoking by PON1 Q192R genotypes remained significant after considering the effects of HDL, age, and sex (χ2 = 80.81, df = 10, P < 0.001; Nagelkerke = 0.309; the number of correctly classified cases was 76.7%). Smoking per se, plasma PON1 activity, and mood disorders were not significant in this analysis. Entering unipolar depression and bipolar disorder versus no mood disorders as an explanatory variable (instead of mood versus no mood disorders) showed that these mood disorders had no significant impact (Wald = 3.42, df = 2, P = 0.181). Entering use of any medications as an additional explanatory variable showed that the use of medications was inversely associated with MetS (Wald = 5.38, df = 1, P = 0.020, B = 0.494, 95% lower and upper confidence interval: 0.272 and 0.897, respectively) and that the effects of PON1 Q192R genotypes and the interaction PON1 Q192R genotype by smoking remained significant. Adjusting for other putative predictors showed that these were not significant and that the effects of PON1 genotype and the smoking by genotype interaction remained significant, i.e. ethnicity (Wald = 0.29, df = 1, P = 0.587); use of alcohol (Wald = 0.79, df = 1, P = 0.375); marital status (Wald = 1.95, df = 1, P = 0.162); years of education (Wald = 0.09, df = 1, P = 0.770), and use of statins (Wald = 0.26, df = 1, P = 0.609).
Interaction smoking by PON1 Q192R genotypes
In order to further examine the interaction between smoking and PON1 Q192R genotype, we performed additional logistic regression analyses in QQ, QR, and RR carriers and in smokers and non-smokers separately. We found that smoking was a significant explanatory variable increasing the odds of the MetS in QQ carriers (Wald = 9.91, df = 1, P = 0.002), but not in QR (Wald = 0.68, df = 1, P = 0.409) or RR carriers (Wald = 0.63, df = 1, P = 0.428). shows the outcome of logistic regression analyses with the same variables as the second regression in but performed in non-smokers and smokers separately. In non-smokers, we found that the PON1 genotypes QQ and QR and HDL cholesterol were inversely associated with MetS (χ2 = 57.84, df = 7, P < 0.001; Nagelkerke = 0.397; the number of correctly classified cases was 83.0%). In smokers, we found that HDL cholesterol was inversely associated with MetS, while age showed a positive relationship (χ2 = 23.41, df = 7, P = 0.001; Nagelkerke = 0.207; the number of correctly classified cases was 72.0%).
Table 4. Results of bivariate logistic regression analyses with MetS as dependent variable and the listed variables as explanatory variables and performed in non-smokers and smokers separately
BMI, obesity, and overweight versus the MetS
Entering BMI as an additional explanatory variable in the full logistic regression depicted in showed that BMI was significantly associated with MetS (Wald = 27.43, df = 1, P < 0.001, B = 1.187, 95% lower and upper confidence interval: 1.113 and 1.265, respectively) and that the effects of PON1 Q192R genotypes and the interaction PON1 genotype by smoking remained significant after adjusting for BMI. In order to delineate whether the PON1 Q192R genotypes may predict BMI, we carried out a multinomial regression analysis with obesity (BMI ≥ 30) and overweight (BMI between 25 and 30) as dependent variables (reference group: subjects with BMI < 25). shows that two variables were significant in predicting obesity or overweight (χ2 = 45.48, df = 20, P = 0.001; Nagelkerke = 0.147), i.e. HDL cholesterol was significantly and inversely associated with overweight (Wald = 14.11, df = 1, P < 0.001, B = 0.961) and obesity (Wald = 9.53, df = 1, P = 0.002, B = 0.961) and male gender was significantly and negatively associated with obesity (Wald = 5.89, df = 1, P = 0.015, B = 0.400), but not overweight (Wald = 0.98, df = 1, P = 0.322).
Table 5. Multinomial regression analysis with obesity, i.e. BMI ≥30 and overweight, i.e. BMI between 25 and 30, as dependent variables and the listed variables as explanatory variables
Discussion
The first major finding of this study is that PON1 Q192R functional genotypes were significantly associated with the MetS. We observed that the QQ and QR genotypes were protective and decreased the odds of having the MetS and thus that RR carriers are more likely to be diagnosed with the MetS. These findings are in accordance with a previous report that the RR allele is significantly associated with an increased risk of the MetSCitation26 and with the a priori hypothesis that the RR genotype may confer an increased risk of MetS. For example, a significant association between the R allele or the RR genotype and increased risk for CVD has been established by Liu et al.Citation24 PON1 RR/QR genotypes and especially the RR genotype are associated with vascular disease, such as stroke and myocardial infarction.Citation48 In hypertensive individuals, the 192 R allele augments the risk of stroke.Citation49 In another study, carriers of the RR (odds ratio 16.24 (6.41–41.14)), but also the QR (odds ratio 2.73 (1.57–4.72)) genotypes had a significant higher risk of coronary artery disease.Citation50 Also in an Iranian study, the PON1 R allele was found to be an independent risk factor for coronary artery disease.Citation51 The PON1 R allele and RR genotype are increased in patients with coronary artery disease.Citation52 In other studies, however, the PON1 Q allele was associated with significantly higher LDL and a worse outcome of CVD.Citation53 Individuals with the QQ genotype have a higher risk of all-cause mortality and cardiac events than RR and QR individuals.Citation54
Our findings that plasma PON1 activity is not lowered in individuals with MetS as compared to those without MetS is not in agreement with previous findings showing lowered PON1 activity in individuals with the MetS.Citation18–Citation23 However, these discrepancies may be explained by differences in PON1 assays. Q192R polymorphism is known to affect PON1 catalytic activity in a substrate-dependent manner.Citation47,Citation55 For example, R192 alloforms have a greater catalytic activity for hydrolysis of paraoxon and chlorpyrifos oxon than Q192 alloforms, Q192 alloforms show a higher activity against some nerve agents, and both alloforms show the same activity against PA and diazoxon.Citation47,Citation55 In our study, we measured PON1 total catalytic (hydrolysis) activity against PA, which at the low salt concentrations used here is not affected by PON1Q192R polymorphism.Citation47,Citation55 Previous studies in the MetS measured paraoxonase or arylesterase activity or PON1 concentrations using enzyme-linked immunosorbent assay methods.Citation20–Citation22 Moreover, PONs are primarily lactonases/lactonizing enzymes, with overlapping substrates and distinctive substrate specificities, although their natural substrates are not well characterized.Citation56 A recent report showed that – until more specific PON1 activity assays employing natural substrates are developed – results of clinical studies may differ depending on the assays that were employed.Citation57
We found that current smoking and especially severe nicotine dependence (according to scores on the Fagerström Nicotine Dependence Scale) and use of more than 31 cigarettes per day were significantly associated with the MetS. These findings are in accordance with previous studies showing a significant association between smoking and the MetS.Citation27–Citation29 Slagter et al.Citation29 additionally found a dose-dependent association between current tobacco use and the MetS. In our study, however, the relationship between tobacco consumption and the MetS was present for heavy tobacco use only, defined as more than 31 cigarettes per day.
The second major finding is that the abovementioned effects of smoking predicting the MetS could be attributed to the interaction between smoking and the PON1 genotypes. Thus, the effects of smoking increasing the odds of MetS were confined to QQ individuals and could not be detected in QR and RR individuals. These results corroborate those of a previous study showing that smoking was significantly associated with a higher risk for myocardial infarction only among QQ homozygotes.Citation58 In another study, smoking increased the risk of ischemic stroke in QQ homozygotes slightly more than in QR + RR individuals, i.e. odds ratio = 2.84 versus 2.33, respectively.Citation49 Such effects may be explained by pathophysiological factors, such as findings that the Q isoform confers reduced protection against oxidation of LDL and HDL.Citation25 Thus, smoking could enhance lipid peroxidation especially in QQ carriers thereby increasing the odds of the MetS and thus CVD in those individuals. Interestingly, a significant interaction between smoking and PON1 genotypes on coronary heart disease risk was also established for the PON1 L55M polymorphism.Citation59 Interestingly, when MetS and diabetes are present PON1-192RR is associated with an increased risk of coronary stenosis especially in smokers.Citation60 All in all, the results show that MetS risk associated with smoking may be attributable to interactions with PON1 Q192R polymorphisms.
In this study, we found no significant association between mood disorders, including unipolar depression and bipolar disorder, and the MetS. A recent meta-analysis performed on 29 cross-sectional studies showed that depression and the MetS are significantly associated with a pooled estimate of 1.42 (95% confidence intervals of 1.28–1.57).Citation3 These discrepancies may perhaps be explained by diagnostic issues, i.e. we used formal DSM-IV diagnosis of mood disorders, while Pan et al.Citation3 showed that the association between depression and the MetS was stronger for self-rated depression (including also subsyndromal depression) than for diagnosis of major depression based on structural interviews. Nevertheless, cohort studies also showed that MetS significantly predicts future clinical depression and that depression may predict future MetS.Citation3 In line with this, reciprocal relationships appear to exist between depression and CVD, depression and diabetes, and depression and obesity.Citation61,Citation62 The bidirectional relationships between mood disorders and the MetS may be explained by shared environmental risk as well as disorders in lipid metabolism, including lowered HDL cholesterol, and activation of immune-inflammatory and O&NS pathways (see the Introduction section).
Limitations of the study are the relatively small sample size of the study, the lack of a more complete PON1 genotyping, and the lack of a more complete evaluation of PON1 activity by means of assays on other substrates, including paraoxon and the newer substrate 5-thiobutyl butyrolactone. Another limitation of the study is that reports derived from ‘association studies constitute tentative knowledge and must be interpreted with caution’.Citation63
In conclusion, this study suggests that the MetS is associated with PON1 Q192R genotypes and a smoking by genotype interaction, but not with lowered PON1 total catalytic activity against PA. The QQ and QR genotypes appear to be protective while smoking by QQ genotype interactions increases the risk of MetS. Severe nicotine dependence and heavy smoking are significantly associated with the MetS, but these effects are no longer significant after considering the effects of PON1 Q192R genotypes and smoking by genotype interactions. The effects of smoking increasing the risk of the MetS appear to be confined to QQ carriers.
Disclaimer statements
Contributors H.O.V. and S.O.V.N. designed the study and collected all data, while all authors contributed equally to the writing up of this paper.
Funding M.B. is supported by a NHMRC Senior Principal Research Fellowship and E.G.M. by a senior fellowship from Fundação Araucária/SETI.
Conflicts of interest None.
Ethics approval The study was approved by the Institutional Review Board of the UEL (number 250/2010) and all subjects gave written informed consent prior to participating in this study.
Acknowledgements
The authors would like to gratefully acknowledge the Health Sciences Graduation Program at State University of Londrina, Brazil, the Ministry for Sciences and Technology of Brazil (CNPq), Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES), and the IMPACT Strategic Research Center, Deakin University, Geelong, Australia.
References
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709–16.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640–5.
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, et al. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care 2012;35(5):1171–80.
- Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol 2006;47:1093–100.
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet 2005;366(9491):1059–62.
- Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation 2004;109(6):706–13.
- He Y, Jiang B, Wang J, Feng K, Chang Q, Zhu S, et al. BMI versus the metabolic syndrome in relation to cardiovascular risk in elderly Chinese individuals. Diabetes Care 2007;30(8):2128–34.
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–28.
- Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 2010;1801:209–14.
- Bryan S, Baregzay B, Spicer D, Singal PK, Khaper N. Redox-inflammatory synergy in the metabolic syndrome. Can J Physiol Pharmacol 2013;91(1):22–30.
- Venturini D, Simão AN, Scripes NA, Bahls LD, Melo PA, Belinetti FM, et al. Evaluation of oxidative stress in overweight subjects with or without metabolic syndrome. Obesity (Silver Spring) 2012;20(12):2361–6.
- Litvinov D, Mahini H, Garelnabi M. Antioxidant and anti-inflammatory role of paraoxonase 1: implication in arteriosclerosis diseases. N Am J Med Sci 2012;4(11):523–32.
- Razavi AE, Ani M, Pourfarzam M, Naderi GA. Associations between high density lipoprotein mean particle size and serum paraoxonase-1 activity. J Res Med Sci 2012;17(11):1020–6.
- Viram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 1998;101(8):1581–90.
- Furlong CE, Suzuki SM, Stevens RC, Marsillach J, Richter RJ, Jarvik GP, et al. Human PON1, a biomarker of risk of disease and exposure. Chem Biol Interact 2010;187(1–3):355–61.
- Martinelli N, Consoli L, Girelli D, Grison E, Corrocher R, Olivieri O. Paraoxonases: ancient substrate hunters and their evolving role in ischemic heart disease. Adv Clin Chem 2013;59:65–100.
- Ferré N, Feliu A, García-Heredia A, Marsillach J, París N, Zaragoza-Jordana M, et al. Impaired paraoxonase-1 status in obese children. Relationships with insulin resistance and metabolic syndrome. Clin Biochem 2013;46(18):1830–6.
- Sentí M, Tomás M, Fitó M, Weinbrenner T, Covas MI, Sala J, et al. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J Clin Endocrinol Metab 2003;88(11):5422–6.
- Liang KW, Lee WJ, Lee IT, Lee WL, Lin SY, Hsu SL, et al. Persistent elevation of paraoxonase-1 specific enzyme activity after weight reduction in obese non-diabetic men with metabolic syndrome. Clin Chim Acta 2011;412(19–20):1835–41.
- Kappelle PJ, Bijzet J, Hazenberg BP, Dullaart RP. Lower serum paraoxonase-1 activity is related to higher serum amyloid a levels in metabolic syndrome. Arch Med Res 2011;42(3):219–25.
- Martinelli N, Micaglio R, Consoli L, Guarini P, Grison E, Pizzolo F, et al. Low levels of serum paraoxonase activities are characteristic of metabolic syndrome and may influence the metabolic-syndrome-related risk of coronary artery disease. Exp Diabetes Res 2012:231502. doi:10.1155/2012/231502. [Epub 2011 Sep 22]. PubMed PMID: 21960992; PubMed Central PMCID: PMC3179885.
- Tabak O, Gelişgen R, Cicekçi H, Senateş E, Erdenen F, Müderrisoğlu C, et al. Circulating levels of adiponectin, orexin-A, ghrelin and the antioxidant paraoxonase-1 in metabolic syndrome. Minerva Med 2012;103(4):323–9.
- Vávrová L, Kodydková J, Zeman M, Dušejovská M, Macášek J, Staňková B, et al. Altered activities of antioxidant enzymes in patients with metabolic syndrome. Obes Facts 2013;6(1):39–47.
- Liu ME, Liao YC, Lin RT, Wang YS, Hsi E, Lin HF, et al. A functional polymorphism of PON1 interferes with microRNA binding to increase the risk of ischemic stroke and carotid atherosclerosis. Atherosclerosis 2013;228(1):161–7.
- Mackness M, Mackness B. Targeting paraoxonase-1 in atherosclerosis. Expert Opin Ther Targets 2013;17(7):829–37.
- Kordi-Tamandani DM, Hashemi M, Sharifi N, Kaykhaei MA, Torkamanzehi A. Association between paraoxonase-1 gene polymorphisms and risk of metabolic syndrome. Mol Biol Rep 2012;39(2):937–43.
- Rabaeus M, Salen P, de Lorgeril M. Is it smoking or related lifestyle variables that increase metabolic syndrome risk? BMC Med 2013;11(1):196.
- Sun K, Liu J, Ning G. Active smoking and risk of metabolic syndrome: a meta-analysis of prospective studies. PLoS ONE 2012;7(10):e47791.
- Slagter SN, Vliet-Ostaptchouk JV, Vonk JM, Boezen HM, Dullaart RP, Kobold AC, et al. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med 2013;11:195.
- Nunes SO, Vargas HO, Prado E, Barbosa DS, de Melo LP, Moylan S, et al. The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence. Neurosci Biobehav Rev 2013;37(8):1336–45.
- Boemi M, Sirolla C, Testa R, Cenerelli S, Fumelli P, James RW. Smoking is associated with reduced serum levels of the antioxidant enzyme, paraoxonase, in Type 2 diabetic patients. Diabet Med 2004;21(5):423–7.
- Salvi V, Albert U, Chiarle A, Soreca I, Bogetto F, Maina G. Metabolic syndrome in Italian patients with bipolar disorder. Gen Hosp Psychiatry 2008;30(4):318–23.
- Foley DL, Morley KI, Madden PA, Heath AC, Whitfield JB, Martin NG. Major depression and the metabolic syndrome. Twin Res Hum Genet 2010;13(4):347–58.
- Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression's multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett 2011;32(1):7–24.
- Jacka FN, Ystrom E, Brantsaeter AL, Karevold E, Roth C, Haugen M, et al. Maternal and early postnatal nutrition and mental health of offspring by age 5 years: a prospective cohort study. J Am Acad Child Adolesc Psychiatry 2013;52(10):1038–47.
- Moylan S, Jacka FN, Pasco JA, Berk M. How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways. Brain Behav 2013;3(3):302–26.
- Moylan S, Eyre HA, Maes M, Baune BT, Jacka FN, Berk M. Exercising the worry away: how inflammation, oxidative and nitrogen stress mediates the beneficial effect of physical activity on anxiety disorder symptoms and behaviours. Neurosci Biobehav Rev 2013;37(4):573–84.
- Berk M, Jacka F. Preventive strategies in depression: gathering evidence for risk factors and potential interventions. Br J Psychiatry 2012;201(5):339–41.
- Maes M, Smith R, Christophe A, Vandoolaeghe E, Van Gastel A, Neels H, et al. Lower serum high-density lipoprotein cholesterol (HDL-C) in major depression and in depressed men with serious suicidal attempts: relationship with immune-inflammatory markers. Acta Psychiatr Scand 1997;95(3):212–21.
- Rabe-Jabłońska J, Poprawska I. Levels of serum total cholesterol and LDL-cholesterol in patients with major depression in acute period and remission. Med Sci Mon 2000;6:539–47.
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606–10.
- Bortolasci CC, Vargas HO, Souza-Nogueira A, Barbosa DS, Moreira EG, Vargas Nunes SO, et al. Lowered plasma paraoxonase (PON)1 activity is a trait marker of major depression and PON1 Q192R gene polymorphism – smoking interactions differentially predict the odds of major depression and bipolar disorder. J Affect Disord 2014;159:23–30.
- Lear SA, James PT, Ko GT, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur J Clin Nutr 2010;64:42–61.
- Del-Ben CM, Vilela JAA, de S Crippa JA, Hallak JEC, Labate CM, Zuardi AW. Confiabilidade da ‘Entrevista Clínica Estruturada para o D.S.M.- IV’ – versão clínica traduzida para o português. Rev Bras Psiquiatr 2001;23(3):156–9.
- Moreno RA, Moreno DH. Hamilton and Montgomery & Asberg depression rating scales. Rev Bras Psiquiatr 1998;25:262–72.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86(9):1119–27.
- Richter RJ, Jarvik GP, Furlong CE. Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ Cardiovasc Genet 2008;1:147–52.
- Baum L, Ng HK, Woo KS, Tomlinson B, Rainer TH, Chen X, et al. Paraoxonase 1 gene Q192R polymorphism affects stroke and myocardial infarction risk. Clin Biochem 2006;39(3):191–5.
- Mahrooz A, Gohari G, Hashemi MB, Zargari M, Musavi H, Abedini M, et al. R-carrying genotypes of serum paraoxonase (PON1) 192 polymorphism and higher activity ratio are related to susceptibility against ischemic stroke. Mol Biol Rep 2012;39(12):11177–85.
- Gupta N, Singh S, Maturu VN, Sharma YP, Gill KD. Paraoxonase 1 (PON1) polymorphisms, haplotypes and activity in predicting cad risk in North-West Indian Punjabis. PLoS ONE 2011;6(5):e17805.
- Vaisi-Raygani A, Ghaneialvar H, Rahimi Z, Tavilani H, Pourmotabbed T, Shakiba E, et al. Paraoxonase Arg 192 allele is an independent risk factor for three-vessel stenosis of coronary artery disease. Mol Biol Rep 2011;38(8):5421–8.
- Bhaskar S, Ganesan M, Chandak GR, Mani R, Idris MM, Khaja N, et al. Association of PON1 and APOA5 gene polymorphisms in a cohort of Indian patients having coronary artery disease with and without type 2 diabetes. Genet Test Mol Biomarkers 2011;15(7–8):507–12.
- Park KW, Park JJ, Kang J, Jeon KH, Kang SH, Han JK, et al. Paraoxonase 1 gene polymorphism does not affect clopidogrel response variability but is associated with clinical outcome after PCI. PLoS ONE 2013;8(2):e52779.
- Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008;299(11):1265–76.
- Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 status as a risk factor for disease or exposure. Adv Exp Med Biol 2010;660:29–35.
- Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res 2005;46(6):1239–47.
- Parra S, Marsillach J, Aragonès G, Rull A, Beltrán-Debón R, Alonso-Villaverde C, et al. Methodological constraints in interpreting serum paraoxonase-1 activity measurements: an example from a study in HIV-infected patients. Lipids Health Dis 2010;9:32.
- Sentí M, Aubó C, Tomás M. Differential effects of smoking on myocardial infarction risk according to the Gln/Arg 192 variants of the human paraoxonase gene. Metabolism 2000;49(5):557–9.
- Robertson KS, Hawe E, Miller GJ, Talmud PJ, Humphries SE. Northwick Park Heart Study II. Human paraoxonase gene cluster polymorphisms as predictors of coronary heart disease risk in the prospective Northwick Park Heart Study II. Biochim Biophys Acta 2003;1639(3):203–12.
- Rejeb J, Omezzine A, Rebhi L, Boumaiza I, Mabrouk H, Rhif H, et al. Association of PON1 and PON2 polymorphisms with PON1 activity and significant coronary stenosis in a Tunisian population. Biochem Genet 2013;51(1–2):76–91.
- Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008;31(12):2383–90.
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010;67(3):220–9.
- Sullivan PF. Spurious genetic associations. Biol Psychiatry 2007;61(10):1121–6.
