Abstract
Objective: This study was aimed to evaluate the cardioprotective potential of combination of T. arjuna and α-tocopherol in isoproterenol induced myocardial injury.
Methods: Wistar albino rats were pre-treated with hydroalcoholic extract of T. arjuna (HETA) and α-tocopherol (100 mg/kg b. w) daily for 30 days. Isoproterenol (ISP, 85 mg/kg b.w) was administered on 28th and 29th days at an interval of 24 hr.
Results: ISP treated rats showed significant increase in lipid peroxidation (MDA), cardiac markers (CK-MB, SGOT, Trop I and LDH), pro-inflammatory cytokine (IL-6, CRP, TNF-α) levels and apoptotic markers (Bcl-2/Bax) as compared to healthy group. Pre-treatment with HETA 100 mg/kg b. w, reduced the elevated levels of these markers and significant effect (p<0.05) were observed with the combination of HETA and α-tocopherol at a dose of 100 mg/kg b. w, which was further confirmed by histopathological studies.
Conclusion: The present study concluded that the combination of α-tocopherol (100 mg/kg b. w) and hydroalcoholic extract of T. arjuna (100 mg/kg b. w) augments endogenous antioxidant compounds of rat heart and also prevents the myocardium from ISP-induced myocardial injury and it may have therapeutic and prophylactic value in the treatment of ischemic heart disease.
Introduction
Ischemic heart disease (IHD) leading to myocardial infarction (MI) is a major cause of morbidity and mortality worldwide.Citation1 MI will be among the most common causes of mortality in India by the year 2015.Citation2 Isoproterenol (ISP), a synthetic β-adrenergic agonist, causes severe myocardial damage in rats, which closely resembles human MI.Citation3,Citation4 The generation of highly cytotoxic free radicals through auto-oxidation of catecholamines plays an important role in ISP-induced cardiac damage.Citation3,Citation4
Development of novel therapies for the management of MI remains a major research thrust globally. There is an urgent need for new drugs that could limit myocardial injury. Medicinal plants may have an important therapeutic window in the management of cardiac ailments. Antioxidants can work most effectively in combination. They can perform a wide spectrum of metabolic activities, free radical scavenging, and preventive actions. Several studies have shown that combinations of vitamins with other antioxidants have synergistic effects.Citation5–Citation8 Terminalia arjuna (T. arjuna) has been used for the treatment of cardiovascular ailments since ancient times.Citation9 The bark of T. arjuna (family Combretaceae) has been mentioned in the ancient Indian medicinal literature to have beneficial effects in heart diseases.Citation10 Due to the strong antioxidant property of the crude bark of T. arjuna, it augments endogenous antioxidant compounds in rat heart.Citation10 Vitamin E (α-tocopherol) is a natural antioxidant that has a characteristic susceptibility to oxidation.Citation11,Citation12 Although there are a large number of studies on α-tocopherol with regard to IHD, its therapeutic effects merit further investigation. In particular, no study has yet addressed the cardioprotective effect of the combination of α-tocopherol and extracts of T. arjuna bark in experimental myocardial ischemia.
The aim of this study was to investigate the preventive effect of the combination therapy of α-tocopherol and hydroalcoholic extract of T. arjuna (HETA) on ISP-induced myocardial damage, biochemical and histopathological alterations.
Materials and methods
Animals
Forty male Wistar albino rats weighing 150–200 g and 10–12 weeks old were used in the study. The study protocol was reviewed and approved by the Institutional Animal Ethical Committee of University College of Medical Sciences, Delhi (IAEC/UCMS/DB-11/2008) and conforms to the Indian National Science Academy guidelines for the use and care of experimental animals in research. Animals were obtained from the central animal house facility of University College of Medical Sciences, Delhi. The rats were housed in polyacrylic cages (38 × 23 × 10 cm3) with not more than four animals per cage. They were housed under standard laboratory conditions with natural light and dark cycles (∼12 hours light/12 hours dark) and maintained at humidity of 55 ± 5% and an ambient temperature of 22 ± 2°C. The animals were allowed free excess to standard pellet diet (Durga Brothers Pvt. Ltd, India) and tap water ad libitum.
Chemicals
ISP was purchased from Sigma Chemical Company, St Louis, USA, α-tocopherol was purchased from Merck, Billerica, MA, USA, and all other chemicals used were of analytical grade. Arjunolic acid was the kind gift of Dr Sayeed Ahmad, Bioactive Natural Product Laboratory, Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India. Creatine kinase MB isoenzyme (CK-MB), serum glutamic oxaloacetic transaminase (SGOT), and lactate dehydrogenase (LDH) assay kits were procured from Spinreact SA, Spain. The enzyme-linked immunosorbent assay (ELISA) kit for Troponin I was from Calbiotech, Spring Valley, CA, USA. ELISA kits for C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNF-α) were purchased from Biovendor (Czech Republic), Calbiotech, and Diaclone (France), respectively. An immunohistostaining kit based on a horseradish peroxidase (HRP) polymer detection system was purchased from Thermo Fisher Scientific, Waltham, MA, USA and primary antibodies (Bax mouse monoclonal IgG2 and Bcl-2 mouse monoclonal IgG1) from Santa Cruz Biotechnology, Dallas, TX, USA.
Preparation of HETA
T. arjuna was obtained from the campus of Institute of Human Behaviour and Allied Seciences (IHBAS), Delhi and authenticated by Dr Sayeed Ahmad, Bioactive Natural Product Laboratory, Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India. The voucher specimen (BNPL/JH/078/2008) was kept for future reference. The bark was crushed in a mixer to a coarse powder (sieve #60) and then extracted with 50% v/v ethanol thrice by macerating the material in 1:20 drug: solvent ratio for 24 hours with occasional shaking at room temperature and sonicating the mixture for 30 minutes before filtration using five to six layers of muslin cloth. The filtrate was first centrifuged and then lyophilized. After lyophilization it became a brown powder. It was stored in a tightly closed bottle, protected from light in the refrigerator at 2–8°C, to be used throughout the experiment. The yield of the hydroalcoholic extract was 18.5% w/w of the dried powder. High-performance thin layer chromatography (HPTLC) fingerprint analysis and estimation of arjunolic acid content was done for quality control/standardization of extract.
Preliminary phytochemical analysis
HETA was subjected to preliminary phytochemical analysis to know the presence/nature of various phytoconstituents viz. saponins, glycosides, alkaloids, tannins, and flavonoids using standard established phytochemical screening methods.Citation13–Citation17
Quantitative analysis of the HETA by HPTLC
The sample was prepared by taking 750 mg of HETA in 25 ml of aqueous acidic solution (HCl 5.0% v/v in water) and refluxed for 1 hour in a water bath. The extract was filtered and taken into a separating funnel. It was then extracted with chloroform and the process repeated three times for complete extraction. The chloroform extracts were pooled and evaporated to dryness. The residue obtained was dissolved in 10 ml of high-performance liquid chromatography (HPLC) grade methanol.
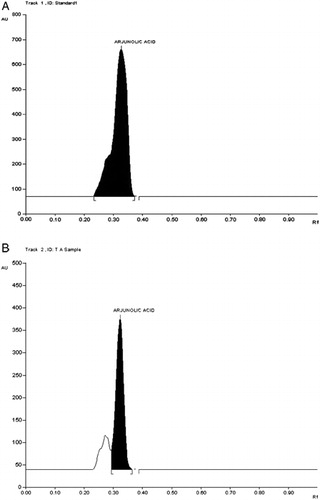
A stock solution of standard arjunolic acid (purity 98%) was prepared in HPLC grade methanol to obtain a 1.0 mg/mlCitation1 solution. The estimation was carried out as per the method described by Singh et al.Citation18 The samples were spotted in the form of bands of width 4 mm using a microliter syringe on pre-coated silica aluminum sheet 60F254 (5.0 × 10 cm2, 0.2 µm thickness) using a Camag Linomat V sample applicator (Switzerland). The plates were pre-washed with methanol and activated at 60°C for 20 minutes prior to chromatography. A constant application rate of 120 nl/second was employed and the space between two bands was 15 mm. The slit dimension was kept at 4 × 0.30 mm2 and 20 mm/second scanning speed was employed. The mobile phase consisted of chloroform: toluene: ethanol (4:4:1, v/v/v) and 10 ml of mobile phase was used per chromatography run. Linear ascending development was carried out in a 10 × 10 cm2 twin trough glass chamber, which had been saturated with mobile phase for 15 minutes. The length of the chromatogram run was 80 mm. After development, thin-layer chromatographic (TLC) plates were dried in a current of air with the help of an air dryer and sprayed with anisaldehyde sulfuric acid reagent and again air dried, then kept in an oven for 10 minutes at 110°C. Densitometric scanning was performed on Camag TLC scanner III operated by Win cats software using a wavelength of 600 nm.
Induction of myocardial ischemia
ISP was freshly prepared in normal saline and injected subcutaneously (s.c.) at a dose of 85 mg/kg body weight (bw) to the rats on the 28th and 29th day at an interval of 24 hours.Citation19–Citation21
Experimental protocol
Animals were divided into five groups, each consisting of eight rats (n = 8).
Group 1 – Healthy control (orally administered normal saline);
Group 2 – Ischemic control (ISP, 85 mg/kg bw);
Group 3 – HETA 100 mg/kg bw + ISP (85 mg/kg bw);
Group 4 – α-tocopherol 100 mg/kg bw + ISP (85 mg/kg bw);
Group 5 – HETA 100 mg/kg bw + α-tocopherol 100 mg/kg bw + ISP (85 mg/kg bw).
The HETA, α-tocopherol, or combined treatment was given for 30 consecutive days once each day using a standard orogastric cannula. On the 28th and 29th days only, rats were given ISP (85 mg/kg bw s.c.) with a 24-hour interval. The healthy control group was not given ISP. α-Tocopherol was dissolved in 5% w/v gum acacia.
Sample collection
Overnight fasted blood samples were collected from the retro-orbital plexus of the animals on days 0, 21, and 30 for biochemical studies. After blood sampling on day 30, the rats were anesthetized with sodium pentobarbital (30 mg/kg, intraperitoneally). To carry out immunohistochemical and histopathological studies, the heart was immediately dissected out, washed in ice-cold saline, and stored in 10% v/v buffered neutral formalin solution.
Biochemical studies
Serum malondialdehyde (MDA) levels were measured as an index of lipid peroxidation using the colorimetric method described by Satoh.Citation22 Lipid peroxides were precipitated from serum with trichloroacetic acid and heated with thiobarbituric acid. The reaction resulted in the formation of a pink-colored chromogen, which was extracted with n-butyl alcohol. Absorbance of the organic phase was determined at 530 nm.
The activity of superoxide dismutase (SOD) in erythrocytes was assayed as described by Marklund and MarklundCitation23 and modified by Nandi and ChatterjeeCitation24 The method is based on the ability of the enzyme SOD to inhibit the auto-oxidation of pyrogallol.
The erythrocyte content of reduced glutathione (GSH) was estimated by the method of Beutler et al.Citation25 The method is based on the development of a yellow color when 5,5′-dithiobis-2-nitrobenzoic acid is added to sulfhydryl compounds.
Estimation of markers of cardiac damage
Markers of cardiac damage (CK-MB, SGOT, and LDH) in serum were estimated spectrophotometrically using commercially available kits. Cardiac Troponin I was measured in serum by a standard ELISA kit.
Estimation of markers of inflammation
Markers of inflammation (IL-6, CRP, and TNF-α) in serum were determined using standard ELISA methods.
Immunostaining for the localization of Bax and Bcl-2 proteins
Anti-Bcl-2 and Bax protein mouse monoclonals were used as the primary antibodies for Bcl-2/Bax immunohistochemical staining. The Ultravision ONE HRP polymer detection system locates the primary antibody with a universal secondary antibody polymer formulation. The amino acid polymer is conjugated to HRP and the Fab fragments of secondary antibody. The polymer complex is visualized with an appropriate chromogen/substrate. Briefly, formalin-fixed paraffin-embedded myocardial sections were subjected to the immunohistochemical procedure for the localization of Bax and Bcl-2 proteins using specific mouse monoclonal primary antibodies. Sections were first blocked and then incubated in primary antibody followed by UltraVision One HRP polymer. The target protein (Bax/Bcl-2) was visualized by incubation in peroxidase substrate (H2O2) using 3,3′-diaminobenzidine (DAB) as the chromogen.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL assay)
Myocardial apoptosis was quantified by the detection of DNA fragmentation using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) technique.Citation26,Citation27 Briefly, the enzyme terminal deoxynucleotidyl transferase was used to incorporate residues of digoxigenin nucleotide into the 3′-OH ends of DNA fragments. The free end of cellular DNA was labeled by incubating the specimens in streptavidin conjugated to HRP and a peroxidase substrate. The signal of terminal TUNEL assay was used to identify apoptotic cells using secondary reaction with antibodies and DAB chromogen. The slides were counterstained in methyl green and total cells and TUNEL-positive cells in the specimens counted.
Histopathological studies
Myocardial tissue fixed in buffered formalin was processed for paraffin embedding, sectioned at 5 µm, and mounted onto microscope slides. These sections were stained with hematoxylin and eosin (H&E), and visualized with a light microscope to study the histoarchitectural changes of the myocardium.
Statistical analysis
The results were expressed as mean ± standard error of the mean (SEM) and statistical differences between mean values were determined by analysis of covariance taking baseline as a covariant. Multiple comparisons among the groups were done using the Bonferroni adjustment method. A value of P < 0.05 was considered statistically significant. In this study out of three timepoints (days 0, 21, 30) only two timepoints (0 and 30) were used, the day 21 data being excluded because ischemia was induced on days 28 and 29. There was no significant difference in values between any days 0 and 21, therefore they were excluded from the study.
Results
Qualitative phytochemical analysis
The freshly prepared HETA was qualitatively tested to check the presence of chemical constituents. These were identified by characteristic color changes using standard procedures. The phytochemical screening of the HETA revealed the presence of tannins, flavonoids, saponins, gums, steroids, alkaloids, reducing sugar, and terpenoids (). It is assumed that these compounds may be responsible for the observed antioxidant and cardioprotective activity of the HETA. Flavonoids and other phenolic compounds of plant origin have been reported as antioxidants and as scavengers of free radicals.Citation28,Citation29 Antioxidants can also exert anti-inflammatory effects.Citation30 The presence of these phytoconstituents in the HETA points towards the pharmacological activities of T. arjuna and is consistent with the claims of traditional users.
Table 1. Qualitative analysis of phytochemicals of HETA
Characterization of HETA by TLC and HPTLC
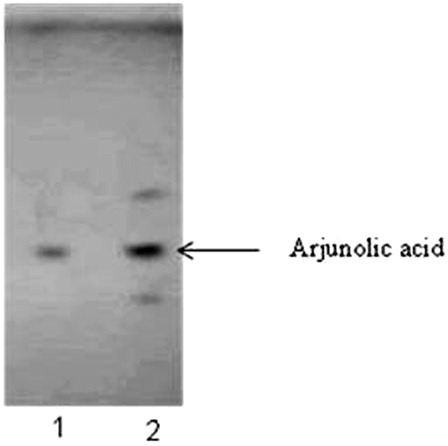
Arjunolic acid, a triterpene present in T. arjuna bark, is protective against acute ISP-induced myocardial changes. The presence of arjunolic acid in HETA was determined by TLC and further characterized by HPTLC. The Rf value for arjunolic acid was 0.44 at 254 nm (). The analysis of HETA showed the presence of 0.394% w/w arjunolic acid ().
A pilot study was done to establish a dose-dependent effect of HETA and α-tocopherol administered at two different doses, HETA (50 and 100 mg/kg bw) and α-tocopherol (50 and 100 mg/kg bw), to ISP-challenged rats. The activity of cardiac marker enzymes and oxidative stress parameters were estimated weekly in rats pre-treated with the combination of HETA and α-tocopherol (100 mg/kg bw). On days 0, 14, and 21, there was no significant differences in the cardiac and oxidative stress parameters. On day 30, there was a significant improvement observed among all groups. Significant effect was observed with the 100 mg/kg bw of HETA and α-tocopherol.
Effect of HETA and α-tocopherol on oxidative stress parameters
There was a significant rise in serum MDA levels in ISP control rats and a significant reduction in erythrocytic GSH and SOD activity as compared to healthy controls. HETA and α-tocopherol at a dose of 100 mg/kg exerted significant effects on oxidative stress markers (MDA, SOD, and GSH), when compared to the ISP control group. However, the maximum significant effects (P < 0.05) were observed with the combination of HETA and α-tocopherol ().
Table 2. Effect of HETA and α-tocopherol on oxidative stress parameters
Effect of HETA and α-tocopherol on cardiac markers
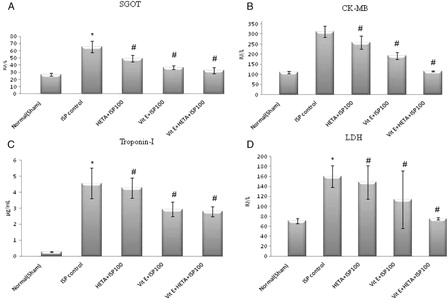
Markers of cardiac damage in serum were elevated during ISP-induced myocardial ischemia in the ISP control group as compared to the healthy group. Pre-treatment with HETA and α-tocopherol, alone and in combination, exerted a significant effect (P < 0.05) as compared to the ISP-treated control group. The serum levels of SGOT, CK-MB, Troponin I, and LDH were elevated during the ischemic episode, but were rectified due to pre-treatment with HETA and α-tocopherol ().
Effect of HETA and α-tocopherol on markers of inflammation
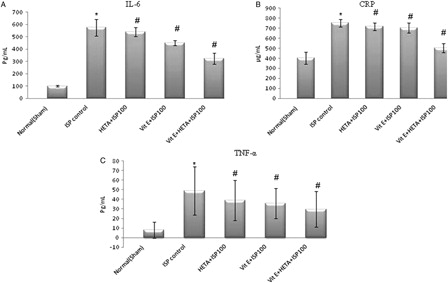
Markers of inflammation, viz. CRP, IL-6, and TNF, were elevated during the ischemic episode following ISP challenge, clearly evident in the ISP control group (). In the healthy group, levels of inflammatory markers were in the normal range (). Pre-treatment with HETA and α-tocopherol individually at a dose of 100 mg/kg produced significant reductions in CRP, IL-6, and TNF levels (P < 0.05). However the combination of HETA and α-tocopherol caused the maximum reduction in markers of inflammation ().
Effect of HETA and α-tocopherol on myocardial apoptosis myocyte Bax/Bcl-2 protein expression and TUNEL positivity
Myocyte Bax protein expression
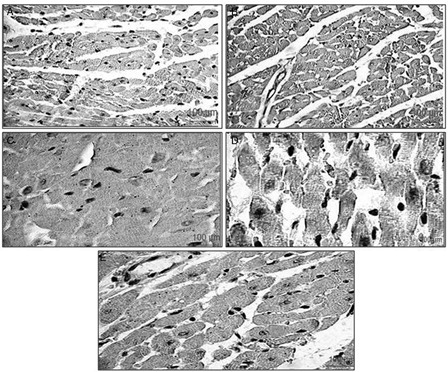
Bax is a pro-apoptotic oncoprotein, which is expressed in ischemic myocardium as evident in the ISP control group (A). Healthy rats show no, or less, immunoreactivity for Bax protein (B). In the HETA (100 mg/kg) pre-treated group, there was reduced Bax expression (C). Pre-treatment with α-tocopherol produced a similar effect to HETA (D). The combination of HETA and α-tocopherol (100 mg/kg) caused a significant inhibition in the expression of Bax protein, which was comparable to healthy controls (E).
Figure 5. Representative photomicrographs of heart ventricular tissue stained for Bax protein from different experimental groups. The localization of Bax protein is indicated by purple positive immunoreactivity. (A) ISP-treated rats, showing increased expression of Bax protein; (B) healthy control rats, showing slight Bax immunoreactivity; (C) HETA-treated rats, showing less Bax expression than ISP-treated control; (D) α-tocopherol-treated rats, showing less Bax expression than ISP-treated control; (E) HETA + α-tocopherol combination, showing attenuated Bax expression compared to ISP-treated control.
Myocyte Bcl-2 protein expression
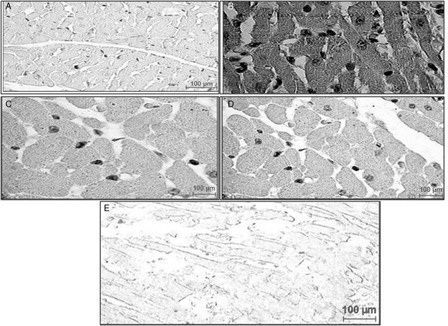
ISP administration resulted in a reduction of Bcl-2 expression, as decreased Bcl-2 immunoreactivity was demonstrated in light microscopic evaluation of sections from the ISP control group (A). Bcl-2 protein was expressed in the myocardium of the healthy control group, as indicated by positive Bcl-2 immunoreactivity in the myocytes (B). Sections from HETA (100 mg/kg) pre-treated rats revealed significant up-regulation in the expression of Bcl-2 (C). The α-tocopherol-treated group also showed improvement in Bcl-2 expression (D). The combination of HETA and α-tocopherol up-regulated Bcl-2 in the myocardium, giving a level comparable to normal myocardium (E).
Figure 6. Immunohistochemical findings of Bcl-2 protein in the rat myocardium from different experimental groups. The localization of Bcl-2 protein is indicated by dark brown positive immunoreactivity. (A) ISP-treated control rats, showing lesser bcl-2 immunoreactivity than healthy controls; (B) healthy control rats, showing positive bcl-2 immunoreactivity in the myocytes; (C) HETA-treated rats, showing up-regulation in the expression of bcl-2 relative to ISP-treated controls; (D) α-tocopherol-treated rats, showing bcl-2 up-regulation relative to ISP-treated controls; (E) HETA + α-tocopherol combination, showing up-regulated bcl-2 expression relative to ISP-treated controls.
TUNEL positivity
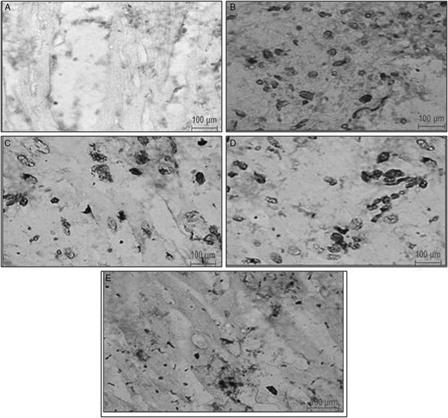
No TUNEL positive cells were observed in the healthy control group (A). In this group, the methyl green staining was indicative of normal cells. However, TUNEL positive cells were seen, as indicated by significant purple to brown staining, in the myocytes of the ischemic control group (B). Pre-treatment with HETA (100 mg/kg bw) significantly reduced the TUNEL positivity (P < 0.05). Pre-treatment with α-tocopherol also reduced the number of TUNEL-positive nuclei as compared to the ischemic control group (C and D). Less TUNEL positivity was observed in rats treated with the combination of HETA and α-tocopherol (E).
Figure 7. Representative photomicrographs of ventricular tissue stained for nick-end labeling (TUNEL) for DNA breaks from different experimental groups. TUNEL-positive nuclei are staining seen as brown to black nuclei. (A) Healthy control rats, showing no TUNEL-positive cells; (B) ISP-treated control rats, showing TUNEL-positive cells; (C) HETA-treated rats, reduced TUNEL positivity relative to ISP-treated controls; (D) α-tocopherol-treated rats, showing reduction in TUNEL positivity relative to ISP-treated controls; (D) HETA + α-tocopherol-treated rats showing maximum reduction in TUNEL positive myocytes relative to ISP-treated controls.
Effect of HETA and α-tocopherol on histology of myocardium
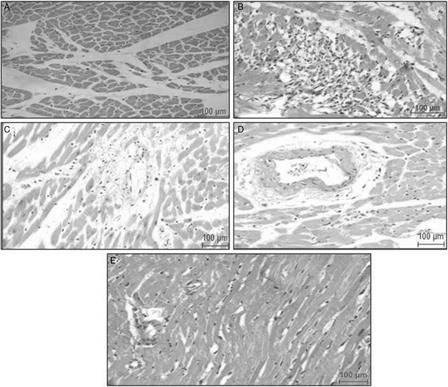
Heart sections from experimental groups were subjected to histopathological study. Microscopic examination of heart sections from the healthy control group showed normal architecture of the myocardium (A). Sections from ISP control rats showed marked focal myonecrosis, loss of striations in myofibrils, vacuolar degeneration, and lymphocytic infiltration (B). HETA-treated rats (100 mg/kg bw) demonstrated structural improvement, decrease in degree of damage, and contraction in myofibrils (C). In addition, lesser vacuolization and inflammation in the myocytes were observed in α-tocopherol-treated rats (100 mg/kg bw) and a reduction in myocardial abnormalities was noted (D); however, the combination of HETA and α-tocopherol showed better improvement in the histopathology and a trend towards normal architecture of the myocardium (E).
Figure 8. Representative photomicrographs of myocardial sections stained with H&E from different experimental groups. (A) Healthy control rats showing normal architecture of myocardium; (B) ISP-treated control rats, showing severe myocardial damage with excessisive inflammatory cell infilteration; (C) HETA-treated rats, showing normal myocardium with less inflammation than in ISP-treated controls; (D) α-tocopherol-treated rats, showing improvement in myocardial architecture relative to ISP-treated controls; (E) HETA + α-tocopherol-treated rats, showing normal myocardium. Figures are representative of at least five separate experiments.
Discussion
The present study was undertaken to screen the cardioprotective potential of HETA and α-tocopherol in an ISP-induced myocardial ischemia model. Phytochemical screening of the HETA revealed the presence of various phytoconstituents, among which flavonoids, steroids, alkaloids, and terpenoids were in abundance. The quantitative analysis of the HETA confirmed the presence of arjunolic acid (0.394% w/w), which has been reported earlier to have a beneficial cardioprotective effect in ISP-induced MI in rats.Citation31,Citation32 Flavonoids and other phenolic compounds of plant origin have been reported as antioxidants and scavengers of free radicals.Citation28,Citation29 α-Tocopherol has been shown to inhibit the progression of IHD in humans and animals.Citation33 Antioxidant therapy involving α-tocopherol has been shown to reduce oxidative stress and improve prognosis in surviving patients with MI.Citation34–Citation37 Therefore in this study, we tried to validate the previously reported protective effect of α-tocopherol alone and test it in combination with HETA in the ISP model.
In the present study, chronic oral administration of HETA and α-tocopherol alone and in combination at 100 mg/kg caused a significant rise in endogenous antioxidants (SOD and GSH) in erythrocytes. However, this increase was maximal with the combination of HETA and α-tocopherol. An increase in MDA (lipid peroxidation marker) is indicative of enhanced oxidative stress. ISP administration raised the serum MDA level significantly, as clearly seen in the ISP control group. The severe myocardial damage in the animals given ISP also can be attributed to peroxidative damage as it previously has been reported that ISP generates lipid peroxides.Citation38 Our present observation is in accordance with the previous findings of increased lipid peroxidation due to ISP challenge, and this was improved with pre-treatment of HETA and α-tocopherol. It was reported previously that medicinal plants and their extracts can stimulate the synthesis of cellular antioxidants.Citation10,Citation39,Citation40 The serum levels of markers of cardiac damage (SGOT, CK-MB, Troponin I, and LDH) were elevated in ISP-treated rats. A significant rise in the levels of diagnostic markers in the serum following ISP administration is an indication of the severity of the necrotic damage to the myocardial membrane.Citation41,Citation42 Thus, a reduction in cardiac markers reflects a reduction in the extent of myocardial damage in rats pre-treated with HETA plus α-tocopherol. To test the influence of HETA and α-tocopherol consumption on inflammation, we measured the serum levels of CRP, IL-6, and TNF, which are established markers of inflammation released after MI. The novel finding of this study is that consumption of the combination of HETA and α-tocopherol reduces serum indices of oxidative stress, cardiac damage, and inflammation.
The combination treatment helped to attenuate apoptosis in the myocardium after MI, indicating that the combination of these supplements is optimal for heart health in this rat model. The infarct-like lesion induced by ISP in rats, originally described by Rona.Citation43 and extensively studied by his group for more than 25 years,Citation44 has provided important information on this model; however, a long-term integrated study is still missing. In the present work, we are the first to offer a long-term study integrating histological, molecular, and biochemical aspects of an experimental model of myocardial ischemic injury induced by ISP in rats, which is clearly evident by the histopathological findings (–8). Two distinct types of cell death in the myocardium, necrosis and apoptosis, have been linked with cardiac damage. Although it has been reported that necrotic cell death leads to destruction of a large group of cells, apoptosis may independently contribute to irreversible myocardial damage.Citation45,Citation46 Induction of apoptosis is implicated in myocardial injury among various cardiovascular diseases.Citation47,Citation48 Cardiomyocyte apoptosis plays an important role in initiation and progression of cardiac diseases. Drugs that effectively and specifically inhibit apoptosis might be useful therapeutic agents for attenuating myocardial injury.Citation49 Hence, the screening of HETA and α-tocopherol for anti-apoptotic activity may be of long-term clinical relevance. In this study, anti-apoptotic evaluation was carried out by means of TUNEL assay and the localization of Bax and Bcl-2 proteins to delineate the involvement of apoptosis in ISP-induced injury. The results of this study demonstrated that ISP administration triggers apoptotic cell death as evident by TUNEL positivity and Bax expression, and reduction in Bcl-2 expression in ISP controls as compared to healthy controls. This observation receives support from earlier studies.Citation50,Citation51 Pre-treatment with the combination of HETA and α-tocopherol reduced apoptotic cell number, consistent with increased Bcl-2 and attenuated Bax expression. It can be suggested that the combination of HETA and α-tocopherol exhibits anti-apoptotic potential due to its strong antioxidant property.
The present study shows that the biochemical and histopathological alterations produced by ISP was attenuated by pre-treatment with the combination of HETA and α-tocopherol. This could be due to the antioxidative effect of this combination against ISP-induced oxidative stress. The results of this study imply that pre-treatment with this combination was effective in reducing the extent of myocardial damage by decreasing lipid peroxidation, myocardial apoptosis, improving antioxidant strength, and hence providing cardioprotection.
Conclusion
An improved myocardial redox state and endogenous antioxidant reserve due to therapy with a combination of HETA and α-tocopherol reduced ISP-induced deleterious changes in the myocardium. The present findings support the cardioprotective potential of the combination of HETA and α-tocopherol therapy with the long-term prospect of being used as an adjunct therapy to prevent IHD.
Disclaimer statements
Contributors SK Shukla performed the experiment, wrote the manuscript, SB Sharma supervised the experiment, checked the manuscript and UR Singh supervised & helped with histopathological study and manuscript writing.
Funding Financial assistance provided by the Central Council for Research in Ayurveda and Siddha (F. No. Z.31014/03/2007/EMR/CCRAS) for this research work is gratefully acknowledged.
Conflicts of interest None.
Ethics approval The study protocol was reviewed and approved by the Institutional Animal Ethical Committee of University College of Medical Sciences, Delhi (IAEC/UCMS/DB-11/2008) and conforms to the Indian National Science Academy guidelines for the use and care of experimental animals in research.
References
- Alwan A, MacLean DR, Riley LM, d'Espaignet ET, Mathers CD, Stevens GA, et al. Monitoring and surveillance of chronic non communicable diseases: progress and capacity in high-burden countries. Lancet 2010;376:1861–8.
- Gilski DJ, Borkenhagen B. Risk evaluation in action for cardiovascular health. Crit Care Nurse 2005;25:26–37.
- Panda VS, Naik SR. Cardio protective activity of Gingko biloba phytosomes in isoproterenol induced myocardial necrosis in rats: a biochemical and histoarchitectural evaluation. Exp Toxicol Pathol 2008;60:397–404.
- Kannan MM, Quine SD. Ellagic acid ameliorates isoproterenol induced oxidative stress: evidence from electro cardiological, biochemical and histological study. Eur J Pharmacol 2011;659:45–52.
- Kagan VE, Serbinova EA, Forte T, Scita G, Packer L. Recycling of vitamin E in human low density lipoproteins. J Lipid Res 1992;33:385–97.
- Yogeeta SK, Balaji HBR, Gnana pragasam A, Senthilkumar S, Subhashini R, Devaki T. Attenuation of abnormalities in the lipid metabolism during experimental myocardial infarction induced by isoproterenol in rats: beneficial effect of ferulic acid and ascorbic acid. Basic Clin Pharmacol Toxicol 2006;98:467–72.
- Punithavathi VR, Prince SM. Combined effects of quercetin and α-tocopherol on lipids and glycoprotein components in isoproterenol induced myocardial infarcted Wistar rats. Chem Biol Interact 2009;181:322–7.
- Pour ZM, Zal F, Monabati A, Vessal MO. Protective effects of a combination of quercetin and vitamin E against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Hepatol Res 2008;38:385–92.
- Dwivedi S. Terminalia arjuna Wight & Arn – a useful drug for cardiovascular disorders. J Ethnopharmacol 2007;7:114–29.
- Gauthaman K, Maulik M, Kumari R, Manchanda SC. Effect of chronic treatment with bark of Terminalia arjuna: a study on the isolated ischemic-reperfused rat heart. J Ethnopharmacol 2001;75:197–201.
- Smolarek AK, Suh N. Chemopreventive activity of vitamin E in breast cancer: a focus on γ and δ tocopherol. Nutrients 2011;3:962.
- Sen CK, Khanna S, Roy S. Tocoterienol: the natural vitamin E to defend the nervous system. Ann N Y Acad Sci 2004;1031:127.
- Trease GE, Evans WC. Pharmacognosy. 13th ed. English Language Book Society, Britain: Bailliere Tindall; 1989. vol. 378, p. 386–480.
- Evans WC, Evans T. Pharmacognosy. 5th ed. London: Cambridge University Press; 2003. vol. 336, p. 93.
- Harborne JB. Phytochemical methods. London: Chapman and Hall Ltd; 1973. p. 49–188.
- Harborne JB. Phytochemical methods. A guide O modern techniques of plant analysis, Chapman and Hall. J Sci Res 2009;1:393–8.
- Sofowora A. Medicinal plants and traditional medicine in Africa. Ibadan: Spectrum Books; 1993. p. 150.
- Singh M, Kamal YKT, Parveen R, Ahmad S. Development and validation of a stability-indicating HPTLC method for analysis of arjunolic acid in an herbal formulation. J Planar Chromatogr 2011;24:172–5.
- Rona G, Chappel CI, Balazs T, Gaudry R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. Arch Pathol 1959;67:443–55.
- Mohanty I, Arya DS, Dinda A, Talwar KK. Protective effects of Curcuma longa on ischemia-reperfusion induced myocardial injuries and their mechanisms. Life Sci 2004;75:1701–11.
- Goyal SN, Arora S, Sharma AK, Joshi S, Ray R, Bhatia J. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine 2010;17:227–32.
- Satoh K. Serum lipid peroxide in cerebrovascular disorder determined by a new colorimetric method. Clin Chem Acta 1978;90:37–43.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47:469–74.
- Nandi A, Chatterjee B. Assay of superoxide dismutase activity in animal tissues. J Biosci 1988;13:305–15.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882–8.
- Gavrieli Y, Sherman Y, Ben Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992;119:493–501.
- Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res 1993;53:1945–51.
- Van Acker FAA, Schouten O, Haenen GRMM, Van der Vijgh WJF, Bast A. Flavonoids can replace α-tocopherol as an antioxidant. FEBS Lett 2000;473:145–8.
- Van den Berg R, Haenen RMM, Van den Berg H, Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem 1999;66:511–7.
- Geronikaki AA, Gavalas AM. Antioxidant and anti-inflammatory disease: synthetic and natural antioxidants with anti-inflammatory activity. Comb Chem High Throughput Screen 2006;9:425–42.
- Sumitra M, Manikandan P, Dilly AK, Natrajan A, Kedike B, Manohar BM, et al. Experimental myocardial necrosis in rats: role of arjunolic acid on platelet aggregation, coagulation and antioxidant status. Mol Cell Biochem 2001;224:135–42.
- Kumar S, Enjamoori R, Jaiswal A, Ray R, Seth S, Maulik SK, et al. Catecholamine induced myocardial fibrosis and oxidative stress is attenuated by Terminalia arjuna (Roxb.). J Pharm Pharmacol 2009;61:1529–36.
- Verlangieri AJ, Bush MJ. Effect of dl-(±)-of α-tocopherol supplementation on experimentally induced primate atherosclerosis. J Am Coll Nutr 1992;11:131–8.
- Singh RB, Niaz MA, Rastogi SS, Rastogi S. Usefulness of antioxidant vitamins in suspected acute myocardial infarction (the Indian experiment of infarct survival-3). Am J Cardiol 1996;77:232–6.
- McMurray J, Mclay J, Chopra M, Bridges A, Belch JJ. Evidence for enhanced free radical activity in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol 1990;65:1261–2.
- Diaz-Velez CR, Garcia-Castineiras S, Mendoza-Ramos E, Hernandez-Lopez E. Increased malondialdehyde in peripheral blood of patients with congestive heart failure. Am Heart J 1996;131:146–52.
- Stephens NG, Parsons A, Schofield PM. Randomised controlled trail of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996;347:781–6.
- Blasig IE, Blasig R, Lowe H. Myocardial lipid peroxidation during isoproterenol-induced blood flow reduction in rat myocardium. Biomed Biochim Acta 1984;43:171–4.
- Pathania V, Syal N, Hundal MH, Khanduja KL. Geriforte stimulates antioxidant defense system. Indian J Exp Biol 1998;36:414–7.
- Banerjee SK, Dinda AK, Manchanda SC, Maulik SK. Chronic garlic administration protects rat heart against oxidative stress induced by ischemic reperfusion injury. BMC Pharmacol 2002;29:16.
- Manjula TS, Geetha A, Shyamala Devi CS. Effect of aspirin on isoproterenol induced myocardial infarction – a pilot study. Indian J Biochem Biophys 1992;29:378–9.
- Geetha A, Sankar R, Thankamani M, Shyamala Devi CS. α-Tocopherol reduces doxorubicin induced toxicity in rats – histological and biochemical evidences. Indian J Physiol Pharmacol 1990;34:94–100.
- Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol 1985;17:291–306.
- Rona G, Chappel CI, Balazs T, Gaudry R. An infarct like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. Arch Pathol 1959;67:443–55.
- Kajstura J, Chong W, Sarangarajan R, Li P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer-344 rats. Am J Physiol 1996;271:1215–28.
- Bartling B, Holtz J, Darmer D. Contribution of myocyte apoptosis to myocardial infarction. Basic Res Cardiol 1998;93:71–84.
- MacLellan WR, Schneider MD. Death by design. Programmed cell death in cardiovascular biology and disease. Circ Res 1997;81:137–44.
- Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res 1998;82:1111–29.
- Saraste A, Pulkki K, Kallajoki M, Henriksen K. Apoptosis in human acute myocardial infarction. Circulation 1997;95:320–3.
- Zhou B, Wu LJ, Li LH, Tashiro S. Silibinin protects against isoproterenol-induced rat cardiac myocyte injury through mitochondrial pathway after up-regulation of SIRT1. Pharmacol Sci 2006;102:387–95.
- Chen G, Zhou X, Florea S, Qian J. Expression of active protein phosphatase 1 inhibitor-1 attenuates chronic beta-agonist-induced cardiac apoptosis. Basic Res Cardiol 2010;105:573–81.