Abstract
Objective
This study aimed to explore the effect of endurance training on oxidative parameters and mitochondrial function in gastrocnemius and heart muscle.
Methods
Male Wistar rats were trained by running for 6 weeks. In vitro measurements of the rates of hydroxyl radical (•OH) production, oxygen consumption (in either the absence, basal rate (V0), or the presence, maximal rate (Vmax), of adenosine diphosphate), and adenosine triphosphate (ATP) production were made simultaneously in permeabilized fibers. The mitochondrial function was explored after exposure or non-exposure to an in vitro generator system of reactive oxygen species (ROS).
Results
Vmax was not affected by training, but V0 decreased. In conditions of maximal mitochondrial functioning, an increase in ATP rate and a decrease in •OH production occurred simultaneously. In vitro ROS exposure disturbed mitochondrial function, but training modified the vulnerability of Vmax and ATP rate to ROS in different ways.
Discussion
We hypothesize that the part of Vmax devoted to proton leakage was decreased in trained rats, consequently improving ATP synthesis. The data suggest that, after training, there is more efficient use of electrons in respiratory chain energy production, rather than a greater ROS scavenging capacity.
Introduction
The mitochondrion is the main supplier of cellular adenosine triphosphate (ATP) in skeletal and heart muscles, through oxidative phosphorylation.Citation1 During mitochondrial functioning, electron transfer along the respiratory chain leads to the formation of most of the radicals and other reactive oxygen species (ROS) produced in these tissues.Citation2 Of the total mitochondrial O2 consumed, 1–2% is converted to superoxide radicals (O2•−), subsequently leading to the formation of other ROS such as hydrogen peroxide (H2O2) and the potentially harmful hydroxyl radical (•OH). This last radical species may react with many molecules in living cells, interfering with cellular processes including mitochondrial biogenesis and energy metabolism.Citation3
It is known that exercise increases ATP demand, particularly in heart and skeletal striated muscle. The source of ATP depends on the exercise protocol (type, duration, intensity) and the type of muscle fibers recruited. During exercise, many sites of ROS production also depending on the protocol may be activated such as enzymes (xanthine oxidase, NADPH oxidase) and mainly the mitochondrial electron transport chain.Citation4 While acute exercise and high-intensity chronic exercise induce ROS formation that potentially causes damage to cell functions, chronic moderate exercise can lead to adaptive mechanisms decreasing oxidative stressCitation5 and radical leakage at the respiratory chain level.Citation6,Citation7 These mitochondrial mechanisms through which regular exercise exerts beneficial effects, including the relationships between mitochondrial energy metabolism and generation of ROS, are not yet well understood. Training effects are often reported on antioxidant defense mechanisms and sometimes related to changes in ROS production and/or oxidative stress markers, but can be difficult to interpret. Indeed, the results are highly dependent on numerous parameters, such as exercise protocol (type, duration, and frequency), species and strain, age, tissue, and gender.Citation5,Citation8,Citation9 Previous studies investigated the effects of training on H2O2 production at the mitochondrial level and results were often conflicting. Starnes et al.Citation7 reported that exercise training (treadmill 5 days per week, 60 minutes per day for 10 weeks) decreased mitochondrial H2O2 production from cardiac mitochondria of male Fischer 344 rats. Venditti et al.Citation6 also reported a decrease in mitochondrial H2O2 release from gastrocnemius muscle of male Wistar rats after swimming-based training. In contrast, Marcil et al.Citation10 showed that exercise training (treadmill 4 days per week during 10 weeks) in female Sprague-Dawley rats did not modify H2O2 production in myocardial mitochondria. Among the adaptations to repeated prolonged exercise, changes in mitochondrial vulnerability toward ROS can occur, but differ widely between studies. Leichtweis et al.Citation11 showed that decrease in heart mitochondrial respiration by exogenous ROS was more pronounced in male Sprague-Dawley rats after rigorous swim training than in untrained animals. On the contrary, Chandwaney et al.Citation12 reported lower vulnerability to exogenous ROS of muscle mitochondria in trained male Fischer 344 (treadmill 5 days per week for 10 weeks) than in untrained rats.
The data from these previous studies are therefore sometimes conflicting because exercise protocol, rat strain, and tissues analyzed are different. To our knowledge, the present study provides the first investigation of the influence of endurance training in male Wistar rat on mitochondrial respiratory function in both cardiac and skeletal muscle, including ATP generation and oxidative parameters.
The influence of moderate chronic exercise (treadmill 5 days per week, 1 hour per day, 6 weeks) was studied in rat skeletal and cardiac muscle by measuring (1) mitochondrial respiratory function (simultaneous measurements of oxygen consumption, ATP, and •OH release from permeabilized fibers); (2) enzymatic antioxidant defenses and oxidative damage (lipoperoxidation); and (3) the vulnerability of mitochondria function to in vitro ROS exposure.
Methods
Animal care
All experimental procedures were approved by the French Ethics Committee (CEFEA n°74) and the ‘Ministère de l'Enseignement Supérieur et de la Recherche’ under the reference 00560.01.
Wistar male rats (n=16), 6 weeks of age (at their arrival) were obtained from Janvier Breeding Center (Le Genest-Saint Isle, France), for use in this study. They were housed in a 21±1°C room with a 12:12 hours light/dark photoperiod. Water and food (standard rat chow) were provided ad libitum.
Training protocol
Maximal aerobic speed (MAS) was evaluated for each rat at 8.5 weeks on a motor-driven treadmill (0% incline). This protocol consisted of an exercise session where the speed was progressively incremented by 0.2 km/hour and then by 0.1 km/hour every 1.5 minutes. MAS was also evaluated after 3 weeks of training to adapt training intensities.
Rats were randomly assigned to either trained (n=8) or sedentary (n=8) groups. Trained rats ran on a treadmill 5 times per week for 60 minutes per day for 6 weeks, at a speed equivalent to 60–70% of their MAS. Sedentary rats were handed identically to trained rats, except that the treadmill was left turned off.
Forty-eight hours after the last session of exercise training, the rats were anesthetized intramuscularly (ketamine/xylazine: 100/15 mg/kg). After anesthesia, the thoracic cage was opened and the heart removed. Ventricle and gastrocnemius muscle were taken. One part of the tissue was used immediately; the remainder was frozen in liquid nitrogen and stored at −80°C until use.
Permeabilized fibers preparation
The left ventricle and gastrocnemius muscle (middle of left muscle head) were minced into fine pieces (∼5 mg/piece). Fibers were placed in buffer A (10 mM EGTA, 20 mM taurine, 3 mM MgCl2, 0.1 mM MES potassium, 0.5 mM dithiothreitol, 15 mM phosphocreatine, and 5 mM ATP; at pH 7.4) containing saponin (100 µg/ml) to selectively destroy the integrity of the sarcolemma. After 20 minutes of incubation, fibers were washed twice in buffer A for 10 minutes to completely remove the saponin and metabolites. All incubations and washing procedures were carried out with mild stirring at 4°C.Citation13
Mitochondrial respiration measurement
The mitochondrial oxygen consumption rate of control and ROS-exposed fibers (around 20 mg) were measured polarographically using a Strathkelvin 928 6-Channel Oxygen System and a Clark-type electrode in a water-jacketed glass chamber maintained at 37°C and equipped with magnetic stirring. The measurement was carried out in 2 ml of a respiratory medium (20 mM Tris, 150 mM KCl, 0.08 mM EDTA, 10 mM NaH2PO4, and 7.5 mM MgCl2; pH 7.2). First, basal respiration (V0), defined as state 4 in mitochondria preparation, was assessed by adding the Krebs cycle intermediates pyruvate/malate (12 mM/6 mM). Then, maximal adenosine diphosphate (ADP)-stimulated respiration (Vmax), defined as state 3 in mitochondria preparation, was measured by adding 5 mM ADP. The respiratory substrates (pyruvate/malate and ADP) were used at saturating concentrations.Citation14 Mitochondrial respiration rate is expressed in µmol O2/minute/g of tissue.
Rate of ATP production by permeabilized fibers
ATP synthesis in muscle fibers was measured in the respiratory medium in the presence of respiratory substrates (see above), as described by Ouhabi et al.Citation15 and Cambier et al.Citation16 We verified that there was no ATP production after addition of pyruvate/malate (V0 conditions). ATP synthesis was initiated by adding 5 mM ADP (Vmax conditions) and was recorded over 2 minutes as follows: every 1 minute after ADP addition, a 20 µl aliquot was withdrawn, quenched in 100 ml dimethyl sulfoxide, and diluted in 5 ml distilled water. The quantity of ATP was measured by bioluminescence with a Berthold detection system luminometer. Standardization was performed with known quantities of ATP (0−500 nmol) measured under the same conditions. The rate of ATP synthesis (VATP) was calculated using a linear regression. Rates were expressed in µmol ATP/minute/g tissue.
Rate of OH• production by permeabilized fibers
For the other part of permeabilized fibers, •OH radical production (V•OH) was determined with an indirect method previously described by Amérand et al.Citation14 Briefly, salicylic acid is used as an •OH radical trapper, and its hydroxylation gives two stable metabolites (2,3- and 2,5-dihydroxybenzoic acid, DHBA), which are further quantified by high-performance liquid chromatography (HPLC) (Knauer – Smartline Autosampler 3900) coupled with electrochemical detection (Bioanalytical system-LC 4C). The incubation of permeabilized fibers with salicylic acid was performed under the same conditions of temperature and saturated substrate concentrations as the VO2 measurements. V•OH production rate was expressed in ng of DHBA/minute/g tissue.
Malondialdehyde content in heart and gastrocnemius muscles
Malondialdehyde (MDA) content, used as an index of lipoperoxidation, was determined according to a method described by Mortelette et al.Citation17 Muscle was homogenized with a polytron homogenizer in a 2% methanolic solution with 1% butylhydroxytoluene. In the presence of 1% phosphoric acid, a complex forms between MDA and thio-butyric acid (TBA) (7.4 mM) after development of the reaction at 100°C for 30 minutes. The TBA/MDA complex was extracted in n-butanol, then evaporated at 37°C and the residue was re-dissolved in the mobile phase. The TBA/MDA complex was separated by HPLC and detected by UV spectrophotometry (BIO-TEK KONTRON) at 532 nm and expressed in nmol/g tissue.
Antioxidant enzyme activities and protein content in heart and gastrocnemius muscles
Enzyme activities and protein content were determined by UV spectrophotometry (UVIKON XL model) at 37°C.
For antioxidant-enzyme activities and protein content, samples from frozen tissues were placed in an extraction buffer (75 mM TRIS and 5 mM EDTA) at 4°C and pH 7.4 for homogenization with a polytron homogenizer prior to centrifugation. After centrifugation at 12 000 g for 10 minutes at 4°C of the resulting supernatant, activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and the protein content were determined:
| • | Total SOD activity was measured at 480 nm using the method that inhibits the adrenaline–adrenochrome reaction.Citation18 One unit (U) of SOD is equal to the amount of sample needed to cause 50% inhibition compared with the control (100%). SOD activity is expressed in U/mg protein. | ||||
| • | CAT activity was determined at 240 nm through its ability to transform H2O2 into H2O and O2.Citation19 The assay concentration of H2O2 was 10 mM in 75 mM TRIS and 5 mM EDTA buffer at pH 7.4. CAT activity is expressed in μmol H2O2/minute/mg protein. | ||||
| • | GPx activity was assessed at 340 nm with an indirect method adapted from Ross et al.Citation20 Briefly, GPx activity was determined from the decrease of NADPH induced by a coupled reaction with glutathione reductase. GPx activity is expressed in μmol NADPH oxidized/minute/mg protein. | ||||
| • | Protein content was measured by the colorimetric method using the BC Assay Protein Quantitation Kit (Uptima-Interchim, France, #FT-40840A). Cu2+ is reduced to Cu+ by proteins in an alkaline medium. The BC Assay (bicinchoninic acid) chelates Cu+ ions with very high specificity to form a water soluble purple-colored complex. The absorbance of this final Cu+ complex is measured with a spectrophotometer at 562 nm and is directly proportional to the protein concentration expressed in mg/g tissue. | ||||
Citrate synthase activity
Citrate synthase (CS) activity was assessed at 412 nm using DTNB (5,5-dithio-bis-2-nitrobenzoic acid).Citation21 Samples from frozen tissues were placed in an extraction buffer (100 mM TRIS) pH 8.1 at 4°C for homogenization with a polytron homogenizer. Measurement was performed on muscle homogenate at 37°C and expressed in nmol/minute/mg protein.
In vitro ROS exposure
A part of the permeabilized fibers were exposed to exogenous ROS by incubating them with FeCl2/H2O2 according to the Fenton reaction (Fe2++H2O2→Fe3++−OH+•OH). We adapted the method to rat heart and gastrocnemius permeabilized fibers.Citation22 In total, 20 mg of permeabilized fibers (four pieces) were transferred to 1.5 ml of buffer A containing 1 mM FeCl2 and 5 mM H2O2. We determined in preliminary experiments that these concentrations would decrease maximal fiber respiration between 30 and 60%. After 30 minutes of incubation in the dark, fibers were washed twice for 5 minutes in 1.5 ml of buffer A. Permeabilized fibers, which are regarded as the control, were incubated in buffer A without H2O2 or FeCl2. All incubations and washing procedures were carried out with mild stirring at 4°C. Measurement of oxygen consumption and ATP production rate was then determined.
Data analysis
Results are expressed as mean±SEM. Statistical significance was evaluated with unpaired Student's t-tests after testing the distributions for normality (Lilliefors' test). Significance was set at P<0.05.
Results
Effects of training on mitochondrial function
Oxygen consumption of permeabilized fibers
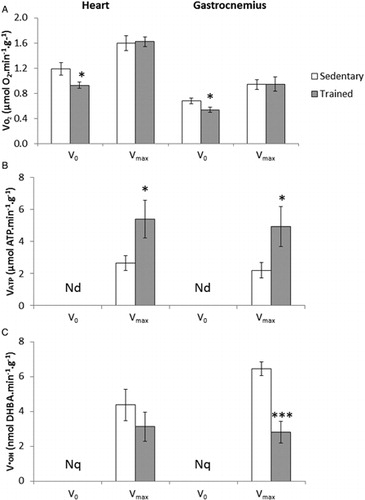
In heart and skeletal muscle, the mitochondrial respiration in the absence of ADP (V0) was significantly lower (P<0.05) in trained rats than in sedentary rats, while maximal ADP-stimulated respiration (Vmax) does not differ between the two groups (A).
Figure 1. Effect of training on (A) oxygen consumption VO2, (B) ATP production VATP, and (C) •OH production V•OH rates in permeabilized heart and gastrocnemius muscle fibers. VO2 and VATP were measured in the absence (V0) and in the presence (Vmax) of ADP and V•OH was measured only in the presence of ADP. Nd = not detected and Nq = not quantified. Values are means ± SEM, *P < 0.05 and ***P < 0.001 relative to sedentary rats, N = 8.
ATP rate (VATP)
The rate of ATP production was undetectable (Nd) in absence of ADP (B). After ADP stimulation, Vmax was significantly higher (P<0.05) in trained rats than in sedentary rats (heart: 5.4±1.2 vs. 2.6±0.5 µmol ATP/minute/g; gastrocnemius: 4.9±1.4 vs. 2.2±0.5 µmol ATP/minute/g; B).
•OH rate (V•OH)
In C, we observe a significantly lower rate of •OH production in the gastrocnemius muscle of trained rats compared with those of sedentary rats (P<0.001). The rate was about 6.4±0.4 nmol DHBA/minute/g for sedentary rats against 2.8±0.6 nmol DHBA/minute/g for trained rats, i.e. a difference of about −56%. In heart tissue, the production of •OH also tended to be about −28% lower in trained rats (not significant).
Effect of training on MDA content and antioxidant enzyme activities
The 6 weeks training did not induce any change in lipid peroxidation. The MDA content did not differ between trained and sedentary rats. As far as tissue protein content and CS activity are concerned, no significant difference was observed between the two groups.
However, shows that training induced lower activity of antioxidant enzyme systems. SOD and GPx activities were significantly lower in heart and gastrocnemius muscles of trained rats, (P<0.01 or better). CAT activity was also lower in gastrocnemius muscle (P<0.01).
Table 1. Effect of training on protein and MDA contents, CS, SOD, CAT, and the GPx activities in heart and gastrocnemius muscle
Effect of training on mitochondrial function after in vitro ROS exposure
As expected, in trained and sedentary rats, in vitro ROS exposure significantly decreased VO2 and VATP (P<0.05).
shows an inverse effect of training on vulnerability of maximal mitochondrial function to ROS exposure. Moreover, in trained rats, VO2max vulnerability is significantly higher than in sedentary rats (39 vs. 30% in heart and 60 vs. 47% in gastrocnemius), P<0.05 (A). In contrast, training induces lower VATP sensitivity significantly in gastrocnemius muscle (34 vs. 50%, P<0.05) and non-significantly in heart (35 vs. 48%, P=0.059) (B).
Figure 2. Effect of training on vulnerability of mitochondrial function to ROS ((A) respiration V0 and Vmax and (B) ATP production VATP) in permeabilized heart and gastrocnemius muscle fibers of sedentary (white histograms, N = 8) and trained rats (gray histograms, N = 8). V0, Vmax, and VATP were measured after 30 minutes of incubation in buffer A containing H2O2+FeCl2 for treated fibers and in buffer A alone for untreated fibers. The change in respiratory and ATP rates is expressed relative to fibers without H2O2 and FeCl2 according to this formula: [(VTreated−VUntreated)/VUntreated] × 100. Values are means ± SEM. *P < 0.05 relative to sedentary rats. Nd = not detected.
![Figure 2. Effect of training on vulnerability of mitochondrial function to ROS ((A) respiration V0 and Vmax and (B) ATP production VATP) in permeabilized heart and gastrocnemius muscle fibers of sedentary (white histograms, N = 8) and trained rats (gray histograms, N = 8). V0, Vmax, and VATP were measured after 30 minutes of incubation in buffer A containing H2O2+FeCl2 for treated fibers and in buffer A alone for untreated fibers. The change in respiratory and ATP rates is expressed relative to fibers without H2O2 and FeCl2 according to this formula: [(VTreated−VUntreated)/VUntreated] × 100. Values are means ± SEM. *P < 0.05 relative to sedentary rats. Nd = not detected.](/cms/asset/9061841b-2369-47c7-8772-0a305146240b/yrer_a_11664138_f0002_b.jpg)
Discussion
The major aim of this study was to investigate the effects of moderate endurance training on ROS production in relation to mitochondrial function in both cardiac and gastrocnemius muscle of rat, and to examine their vulnerability to ROS after training. The use of permeabilized muscle fibers has the advantage to assess, in situ, the mitochondrial function since isolation of mitochondria tends to alter their integrity.Citation23 Whatever the type of exercise, the mitochondrial electron transport chain is considered an important source of ROS in muscle fibers. In such conditions, enhanced ATP requirements increases oxygen flux in the heart and skeletal muscle mitochondria, and may favor a high rate of free radical leakage.Citation24 Oxygen consumption may reach 10−20 times the systemic levels and as much as 100−200 times the skeletal muscle levels, resulting in high increases in mitochondrial electron flux.Citation5 In the same manner, myocardial tissue can increase its oxygen consumption by 10 times during vigorous exercise; high oxygen flux in mitochondria may also lead a higher rate of leakage of free radicals.Citation25 It is clearly established that chronic exercise produces significant adaptations both in skeletal and cardiac muscle, which improve the oxidative capacity. However, training may also elicit adaptation that protects tissues from further ROS-induced damageCitation11. In many studies, training has some positive gene modulator effects on enzymatic and non-enzymatic antioxidant systems,Citation26 but such effects cannot be generalized because the intensity of endurance training and the duration of the session may influence the adaptions of these antioxidant systems.
The first main result of the present study is that training induces a concomitant increase in ATP production rate and decrease in •OH production (in conditions of maximal mitochondrial functioning, Vmax) suggesting an improvement in energy efficiency. The second main observation is the decrease in activity of the antioxidant defense system in both cardiac and gastrocnemius muscles of trained rats but with no evidence of oxidative stress, estimated by MDA quantification.
Training significantly decreases the non-ADP-stimulated respiration (V0) around −23% in heart and −17% in gastrocnemius (). V0 is due to back leakage of protons into the mitochondrial matrix through other ion inner membrane channels rather than through F0F1ATP synthase. This proton backflow can occur either through the lipid bilayer or can be facilitated by the inducible uncoupling proteins (UCP). It is known that this non-ATP productive pathway could account for around 20−25% of basal metabolic rate.Citation27 In perfused rat muscle, it was measured at 35% in contracting preparations and 50% in resting muscle.Citation28 So, the observed decrease in V0 can be interpreted as a decreased permeability of the inner mitochondrial membrane to protons, which could lead to the passage of protons through ATP synthase and so increase the coupling of oxidative phosphorylation. Lumini-Oliveira et al.Citation29 also showed decreased permeability of the inner mitochondrial membrane, not in permeabilized fibers but in mitochondria, which was estimated by the measurement of the ratio between uncoupled respiration (induced by cyanide m-chlorophenylhydrazone addition) and the respiratory rate in the presence of oligomycin. Boss et al.Citation30 showed a decrease in UCP3 and UCP2 in heart and skeletal muscle of rat after endurance training, and concluded that there was a higher level of metabolic efficiency. Therefore, in our study, a decrease in V0 after training could mean that oxidative efficiency is related not to quantitative adaptations such as an increase in mitochondrial mass but to a change in the permeability of the inner mitochondrial membrane.Citation31 We did not observe differences in CS activity in gastrocnemius and heart muscles with training (). The determination of CS activity is classically used as a marker of mitochondrial biogenesis. Pinho et al.Citation9 also reported no change in CS activity in mixed gastrocnemius muscle in male Wistar rats treadmill-trained for 12 weeks. In conditions of moderate training, unchanged CS activity is also frequent in heart, while CS activity is often increased in slow twitch skeletal muscle such as soleus.Citation32 Siu et al.Citation33 suggested that heart tissue, may be because its continual activity, has a sufficient pre-existing oxidative capacity to supply the energy requirement during exercise.
Maximal ADP-stimulated respiration (Vmax) of permeabilized fibers was not affected by endurance training (). Other studies using different types of exercise in rat have also shown no difference in Vmax or state 3 in mitochondria. Servais et al.Citation34 and Judge et al.Citation35 reported unchanged state 3 respiration in quadriceps muscle and in heart, respectively, of rats that performed voluntary wheel-running exercise. Venditti et al.Citation6 also reported unaffected mitochondrial oxygen consumption in skeletal muscle after swimming training and in heart after running training, respectively. More generally, however, improvement in maximal ADP-stimulated respiration in permeabilized fibers or state 3 in mitochondria are increased in humans and animals. For example, 14 weeks of endurance treadmill running induced a significant increase in state 3 (12% with pyruvate/malate as substrates) and in respiratory control ratio (44%) equivalent to state 3/state 4 ratio in skeletal muscle of Wistar rats.Citation29 These results suggest that different adaptions occur at the mitochondrial level and/or reflect the variability of the protocols used.
In the present study, the respiratory data show that the acceptor control ratio (Vmax/V0), a parameter representing the degree of coupling between oxidation and phosphorylation, is improved by around 17% after training in heart and gastrocnemius muscle. The hypothesis of an improvement in the electron transport to phosphorylation coupling is also supported by measurements of ATP and ROS production.
The rate of ATP release by permeabilized muscle fibers, measured in conditions of maximal mitochondrial functioning is significantly higher in trained rats (32% in the gastrocnemius muscle and 52% in the heart muscle) (). El Midaoui et al.Citation36 also found an increase in ATP production rate of 33% in the gastrocnemius muscle after 10 weeks endurance training and Wibom et al.Citation37 showed a 50% increase in human vastus lateralis after 6 weeks of endurance training.
It is also important to take into account some methodological considerations when interpreting our data. The method used in our study to estimate the ATP production rate in permeabilized fibers does not assess true mitochondrial ATP synthesis. The measured ATP is the result of the overall yield between ATP synthesis and hydrolysis. The method used measures the maximal in vitro capacity for ATP production. Some precautions were taken to ensure that the major part of ATP measured was related to oxidative phosphorylation. Oxygen consumption rate (VO2) was measured in parallel to ATP measurement, as shown in . Indeed, we observe that the measured VATP occurs when ADP is added to stimulate oxidative phosphorylation. The absence of VATP in conditions of basal respiration (V0) shows that the permeabilization procedure eliminated intracellular ADP. Other sources or consumers of ATP than mitochondria are potentially present, although the permeabilization procedure eliminates most metabolites and cytosolic enzymes, such as phosphocreatine or glycolytic enzymes, adenylate kinase, ATP hydrolases and synthesis enzymes.Citation15 In particular, because several ATP hydrolases are located in the membrane and perhaps not lost with the permeabilization, EDTA was used to chelate cytosolic magnesium and so inhibit the activity of these enzymes. In our study, the increase in ATP rate with training was not associated with an enhancement in oxygen consumption rate, which could at first appear surprising. However, we can hypothesize that for the same Vmax, the proportion that is devoted to proton leakage is decreased and ATP synthesis is thus improved. Other mechanisms not explored in the present study can be involved in the increase in ATP rate. For example, training may increase the ADP/ATP exchange at the mitochondrial levelCitation38 and induce changes in the activity of mitochondrial creatine kinase.Citation39 These mechanisms may increase the ADP diffusion into mitochondrial matrix for rephosphorylation and activate respiration.
Concerning ROS, endurance training decreases •OH production from skeletal and cardiac muscle permeabilized fibers measured in conditions of maximal mitochondrial functioning (). These results agree with the decreased mitochondrial H2O2 release obtained by Venditti et al.Citation6 in gastrocnemius muscle of Wistar rat after a swimming protocol (5 days per week for 10 weeks). Long-term voluntary wheel running or exercise training (running training for 16 weeks) also diminished H2O2 production from heart mitochondria in Fisher rats.Citation7,Citation35
Classically, a decrease in ROS production (•OH or H2O2) is related to enhanced antioxidant enzyme activities or to lowered superoxide generation. In our present study, SOD and GPx activities, expressed in mg protein, are lower in trained rats compared with sedentary ones in cardiac and skeletal muscle (). CAT activity is also decreased in gastrocnemius but is stable in heart. These results are unusual because in most studies using training the activity of these enzymes is upregulatedCitation40 or unchanged.Citation26 The decrease in antioxidant enzymes activity could be an adaptation to the lower ROS production related to a decrease in electron leak at the mitochondrial level. It is known that the extent of adaptation of antioxidant enzymes depends on the training intensity, duration, and type of muscle. A decrease in SOD activity has been reported with voluntary exercise.Citation41 Gul et al.Citation24 also showed a decrease in the activities of GPx and reductase in the heart of rats that had performed endurance training for 8 weeks.
Decrease in antioxidant enzyme activities could make the heart and gastrocnemius muscle vulnerable to oxidative stress, but no increase in lipoperoxidation (MDA) was observed after training (); this result is also consistent with that obtained by Gul et al.Citation24 But it remains difficult to confirm that no oxidative stress occurred because protein or DNA damage was not analyzed.
Whatever the tissue considered and the rat activity level (sedentary or trained), in vitro ROS exposure disturbed mitochondrial function (). Incubation of the fibers in the presence of ROS decreased not only Vmax but also V0 and VATP in both heart and skeletal muscle. The relative decrease in respiratory rate VO2 was greater in gastrocnemius than in heart, suggesting a better antioxidant protection of this latter (although this was not confirmed by antioxidant enzymatic enzyme measurements in our study, see ). VATP rate sensitivity to ROS was similar in both tissues. As shown in , training modifies mitochondrial sensitivity to ROS in gastrocnemius muscle and in heart (tendency, P=0.059) but differently from Walsh et al.Citation22 These previous authors showed that the vulnerability of mitochondrial respiration to ROS was unchanged after endurance training in skinned fibers. In our study, the sensitivity of V0 to ROS was not modified by training, in contrast to Vmax that was more inhibited by in vitro ROS exposure. In parallel, VATP in muscle fibers from trained rats showed a greater resistance to ROS exposure than those from sedentary rats, suggesting that energy production was better protected after training.
It is interesting to note that training does not seem to affect the different components of the respiratory chain in the same manner. It is well known that some compounds involved in the mitochondrial function are particularly ROS sensitive; the most frequently cited being ATP/ADP translocase, F0-F1 ATPase, UCP 2 and 3, but also the permeability transition pore.Citation16 It would be interesting to investigate, using specific inhibitors, the activity and/or expression of some of these proteins and of the complexes of the respiratory chain after exposure to ROS. We can speculate that training produces an adaption in muscle which modifies the vulnerability of some specific mitochondrial components to ROS. Leichtweis et al.Citation11 suggested that some components of heart mitochondria chronically exposed to the exercise-induced oxidative stress became more sensitive to ROS exposure. After training, some of these components especially involved in the phosphorylating system (ATP synthase, adenine nucleotide translocase, and phosphate transporters) could be less affected by ROS exposure. Because antioxidant enzymatic activities were lower in trained rats we suggest that other non-enzymatic antioxidants present in the cell, including vitamin E, GSH and ubiquinone could be involved in the oxidative protection. These antioxidants were not examined in the present study, but they could also be modified by endurance training.Citation40 A future investigation of these non-enzymatic antioxidants would be interesting.
The data detailed above, when separately analyzed and compared to the literature, are at first surprising, but once put into perspective lead to a consistent interpretation. In this study performed in Wistar males, endurance training of 6 weeks leads similar responses in permeabilized fibers of heart and gastrocnemius. Under conditions of maximal mitochondrial functioning (ADP-stimulated respiration), a decrease was observed in ROS production (a tendency in heart muscle and a significant effect in gastrocnemius) in parallel to an increase in ATP rate. These data suggest that there is a better use of electrons at the respiratory chain level to produce energy rather than a greater capacity to scavenge O2•− or H2O2, because antioxidant enzyme activities decreased. After training, the unchanged ADP stimulated respiration but a decrease in V0 suggested a modification of proton membrane permeability.
Our results confirm that after endurance training, mitochondria use multiple strategies to reduce free radical leakage and enhance mitochondrial function.Citation42
Disclaimer statements
Contributors All authors contributed equally.
Funding This research was supported by a grant from the Ministry of Higher Education (France).
Conflicts of interest None.
Ethics approval All experimental procedures have been approved by the French Ethics Committee (CEFEA n°74) and the “Ministère de l'Enseignement Supérieur et de la Recherche” under the reference 00560.01.
References
- Yaniv Y, Juhaszova M, Nuss HB, Wang S, Zorov DB, Lakatta EG, et al. Matching ATP supply and demand in mammalian heart: in vivo, in vitro, and in silico perspectives. Ann N Y Acad Sci 2010;1188:133–42.
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82(1):47–95.
- Yoboue ED, Devin A. Reactive oxygen species-mediated control of mitochondrial biogenesis. Int J Cell Biol 2012;2012:403870.
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 2008;88(4):1243–76.
- Silva LA, Pinho CA, Scarabelot KS, Fraga DB, Volpato AM, Boeck CR, et al. Physical exercise increases mitochondrial function and reduces oxidative damage in skeletal muscle. Eur J Appl Physiol 2009;105(6):861–7.
- Venditti P, Masullo P, Di Meo S. Effect of training on H(2)O(2) release by mitochondria from rat skeletal muscle. Arch Biochem Biophys 1999;372(2):315–20.
- Starnes JW, Barnes BD, Olsen ME. Exercise training decreases rat heart mitochondria free radical generation but does not prevent Ca2+-induced dysfunction. J Appl Physiol (1985) 2007;102(5):1793–8.
- Pinho CA, Tromm CB, Tavares AM, Silva LA, Silveira PC, Souza CT, et al. Effects of different physical training protocols on ventricular oxidative stress parameters in infarction-induced rats. Life Sci 2012;90(13–14):553–9.
- Pinho RA, Andrades ME, Oliveira MR, Pirola AC, Zago MS, Silveira PC, et al. Imbalance in SOD/CAT activities in rat skeletal muscles submitted to treadmill training exercise. Cell Biol Int 2006;30(10):848–53.
- Marcil M, Bourduas K, Ascah A, Burelle Y. Exercise training induces respiratory substrate-specific decrease in Ca2+-induced permeability transition pore opening in heart mitochondria. Am J Physiol Heart Circ Physiol 2006;290(4):1549–57.
- Leichtweis SB, Leeuwenburgh C, Parmelee DJ, Fiebig R, Ji LL. Rigorous swim training impairs mitochondrial function in post-ischaemic rat heart. Acta Physiol Scand 1997;160(2):139–48.
- Chandwaney R, Leichtweis S, Leeuwenburgh C, Ji LL. Oxidative stress and mitochondrial function in skeletal muscle: effects of aging and exercise training. Age (Omaha) 1998;21(3):109–17.
- Mortelette H, Moisan C, Sebert P, Belhomme M, Amerand A. Fish as a model in investigations about the relationship between oxygen consumption and hydroxyl radical production in permeabilized muscle fibers. Mitochondrion 2010;10(5):555–8.
- Amérand A, Vettier A, Sebert P, Cann-Moisan C. In vitro effect of hydrostatic pressure exposure on hydroxyl radical production in fish red muscle. Redox Rep 2005;10(1):25–8.
- Ouhabi R, Boue-Grabot M, Mazat JP. Mitochondrial ATP synthesis in permeabilized cells: assessment of the ATP/O values in situ. Anal Biochem 1998;263(2):169–75.
- Cambier S, Benard G, Mesmer-Dudons N, Gonzalez P, Rossignol R, Brethes D, et al. At environmental doses, dietary methylmercury inhibits mitochondrial energy metabolism in skeletal muscles of the zebra fish (Danio rerio). Int J Biochem Cell Biol 2009;41(4):791–9.
- Mortelette H, Amerand A, Sebert P, Belhomme M, Calves P, Moisan C. Effect of exercise training on respiration and reactive oxygen species metabolism in eel red muscle. Respir Physiol Neurobiol 2010;172(3):201–5.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170–5.
- Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 1952;195(1):133–40.
- Ross SW, Dalton DA, Kramer S, Christensen BL. Physiological (antioxidant) responses of estuarine fishes to variability in dissolved oxygen. Comp Biochem Physiol C Toxicol Pharmacol 2001;130(3):289–303.
- Srere P. Citrate synthase: [EC4.1.3.7.Citrate oxaloacetate-lyase(CoA-acetylating)]. Meth Enzym 1969;13:3–11.
- Walsh B, Tonkonogi M, Sahlin K. Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibres. Pflugers Arch 2001;442(3):420–5.
- Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 1998;184(1–2):81–100.
- Gul M, Demircan B, Taysi S, Oztasan N, Gumustekin K, Siktar E, et al. Effects of endurance training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp Biochem Physiol A Mol Integr Physiol 2006;143(2):239–45.
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 2005;115(3):500–8.
- Ascensao A, Ferreira R, Magalhaes J. Exercise-induced cardioprotection – biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. Int J Cardiol 2007;117(1):16–30.
- Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol 2000;35(6–7):811–20.
- Rolfe DF, Newman JM, Buckingham JA, Clark MG, Brand MD. Contribution of mitochondrial proton leak to respiration rate in working skeletal muscle and liver and to SMR. Am J Physiol 1999;276( 3 Pt 1):C692–9.
- Lumini-Oliveira J, Magalhaes J, Pereira CV, Aleixo I, Oliveira PJ, Ascensao A. Endurance training improves gastrocnemius mitochondrial function despite increased susceptibility to permeability transition. Mitochondrion 2009;9(6):454–62.
- Boss O, Samec S, Desplanches D, Mayet MH, Seydoux J, Muzzin P, et al. Effect of endurance training on mRNA expression of uncoupling proteins 1, 2, and 3 in the rat. FASEB J 1998;12(3):335–9.
- Zoll J, Sanchez H, N'Guessan B, Ribera F, Lampert E, Bigard X, et al. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 2002;543( Pt 1):191–200.
- Zonderland ML, Bar PR, Reijneveld JC, Spruijt BM, Keizer HA, Glatz JF. Different metabolic adaptation of heart and skeletal muscles to moderate-intensity treadmill training in the rat. Eur J Appl Physiol Occup Physiol 1999;79(5):391–6.
- Siu PM, Donley DA, Bryner RW, Alway SE. Citrate synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol (1985) 2003;94(2):555–60.
- Servais S, Couturier K, Koubi H, Rouanet JL, Desplanches D, Sornay-Mayet MH, et al. Effect of voluntary exercise on H2O2 release by subsarcolemmal and intermyofibrillar mitochondria. Free Radic Biol Med 2003;35(1):24–32.
- Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, et al. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol 2005;289(6):1564–72.
- El Midaoui A, Tancrede G, Nadeau A. Effect of physical training on mitochondrial function in skeletal muscle of normal and diabetic rats. Metabolism 1996;45(7):810–6.
- Wibom R, Hagenfeldt L, von Dobeln U. Measurement of ATP production and respiratory chain enzyme activities in mitochondria isolated from small muscle biopsy samples. Anal Biochem 2002;311(2):139–51.
- Fernstrom M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol 2004;554( Pt 3):755–63.
- Guerrero K, Wuyam B, Mezin P, Vivodtzev I, Vendelin M, Borel JC, et al. Functional coupling of adenine nucleotide translocase and mitochondrial creatine kinase is enhanced after exercise training in lung transplant skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2005;289(4):1144–54.
- Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, et al. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol 1994;266( 2 Pt 2):375–80.
- Selman C, McLaren JS, Collins AR, Duthie GG, Speakman JR. Antioxidant enzyme activities, lipid peroxidation, and DNA oxidative damage: the effects of short-term voluntary wheel running. Arch Biochem Biophys 2002;401(2):255–61.
- Daussin FN, Rasseneur L, Bouitbir J, Charles AL, Dufour SP, Geny B, et al. Different timing of changes in mitochondrial functions following endurance training. Med Sci Sports Exerc 2012;44(2):217–24.
