Abstract
Objectives
Patients with chronic kidney disease have impaired muscle metabolism, resulting in muscle atrophy. Oxidative stress has previously been identified as a significant contributor to muscle atrophy in other populations, but the contribution in chronic kidney disease is unknown. The aim of this study was to investigate the association between oxidative stress, grip strength, and lean mass in patients with chronic kidney disease.
Methods
This is a cross-sectional study of 152 participants with stage 3 or 4 chronic kidney disease. Outcome measures include grip strength, lean mass, plasma total F2-isoprostanes, inflammation, peak oxygen uptake, and standard clinical measures.
Results
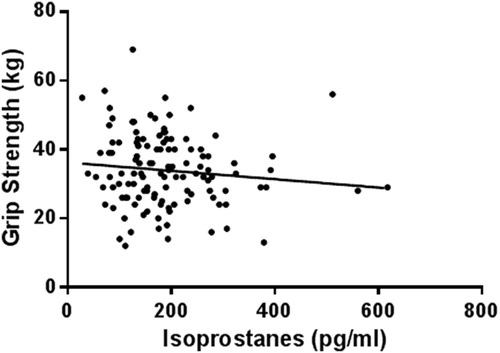
Thirty four (22.4%) chronic kidney disease patients had elevated oxidative stress levels (plasma F2-isoprostanes >250 pg/ml), with 82% of patients below age-predicted grip strength normative values. There was a significant negative association between plasma F2-isoprostanes and grip strength (r = −0.251) and lean mass (r = −0.243). There were no associations with inflammation markers. Multiple linear regression identified plasma F2-isoprostanes as a significant predictor of grip strength independent of other predictors: sex, diabetes status, body mass index, body fat percent, and phosphate (adjusted r2 = 69.5, P < 0.001).
Discussion
Plasma F2-isoprostanes were independently associated with reduced strength in chronic kidney disease patients.
Introduction
Patients with chronic kidney disease (CKD) develop muscle wasting, which significantly influences muscular strength.Citation1 Skeletal muscle atrophy is associated with a three-fold increase in mortality over a 4–6-year period in dialysis patients.Citation2 The aetiology of muscle atrophy in CKD is multi-factorial and associated with metabolic acidosis, excess angiotensin-II, and inflammation, however, to our knowledge the contribution of oxidative stress in pre-dialysis patients has not been studied.Citation1
Oxidative stress occurs when there is a disruption of redox signalling and control pathways.Citation3 A well-documented target of oxidative injury is lipid peroxidation of arichidonic acid, which produces F2-isoprostanes.Citation4 As such, plasma F2-isoprostanes are the gold standard for quantifying oxidative stress.Citation5 Other common assays or biomarkers of oxidative stress in CKD patients include advanced oxidation of protein products, protein carbonyls, γ-glutamyl transpeptidase, and malondialdehyde.Citation4 Protein carbonyls quantify reactive species damaged proteins, however, this assay is not a specific measure of oxidative stress as it also measures glycated proteins and bound aldehyde.Citation4 Reduced total anti-oxidant capacity (TAC) and glutathione peroxidase (GPX) may also indicate a disturbance of the redox signalling pathways. Elevated oxidative stress levels, measured by plasma-free F2-isoprostanes, protein carbonyls, and protein-reduced thiol content, have been reported in moderate-severe CKD.Citation6 Oxidative stress is suggested to be associated with inflammation, endothelial dysfunction, and malnutrition in the uraemic population, thereby synergistically contributing to atherogenecity and risk of a cardiovascular event occurring.Citation7 Despite the multi-factorial nature of oxidative stress in renal patients, it has been suggested that the retention of oxidized solutes is likely a major contributor to the disease process.Citation8 Only one small study has investigated the effects of oxidative stress and muscle atrophy in renal patients. Crowe et al.Citation9 identified that haemodialysis patients (n = 10) had significantly reduced diameter size of type-I and -II muscle fibres when compared to sex-matched controls. However, the authors found no clear association between an oxidative stress marker, serum malonaldehyde, and muscle fibre diameter. Whether an increase in oxidative stress with CKD influences muscle atrophy and strength is yet to be examined.
Grip strength is well recognized as an indicator of overall upper body strengthCitation10 and provides risk estimates similar to those of quadriceps strength.Citation11 It has been suggested that muscle strength is a greater predictor of mortality than muscle mass.Citation11 Moreover, it has been reported that hand grip strength is a reliable measure of lean body mass in both men and women with chronic renal failure.Citation12 Due to the strong validity and accessibility of grip strength measures in the community, this study focused on grip strength as a primary measure of strength.
The aim of this study was to investigate the association between oxidative stress, grip strength, and lean mass in patients with CKD. It was hypothesized that CKD patients would have impaired muscle function, evidenced by grip strength lower than age-predicted normative values. Furthermore, it was hypothesized that oxidative stress would be negatively associated with lean mass and grip strength.
Methods
The data from this study is a cross-sectional baseline analysis of the ‘LANDMARK 3’ study (Longitudinal Assessment of Multiple Discrete Atherosclerotic Risk Factors in Kidney Disease), looking at the effects of a 3-year multidisciplinary lifestyle intervention in CKD. This study included 152 subjects with stage 3 or 4 CKD (modification of diet in renal disease-175; estimated glomerular filtration rate (eGFR) 25–60 ml/min/1.73 m2). Inclusion criteria were: aged 18–75 years and at least one of the following risk factors – blood pressure or lipids not at target, overweight (body mass index (BMI) >25 kg/m2), and poor diabetic control (haemoglobin A1c>7%). Exclusion criteria were: intervention for, or, symptomatic coronary artery disease (within 3 months), current heart failure (New York Heart Association class III and IV) or significant valvular heart disease, pregnant or planning to become pregnant, and life expectancy or anticipated time to dialysis or organ transplant <6 months.
The study protocol was approved by the Princess Alexandra Human Research Ethics Committee (HREC 2007/190), and was registered at http://www.anzctr.org.au (Registration Number ANZCTR12608000337370). All patients gave written, informed consent to participate in this study.
Ethylenediaminetetraacetic acid vacutainers (BD vacutainers, Franklin Lakes, NJ, USA) were used to collect 10 ml venous blood samples following an overnight fast. Samples were stored on ice before being centrifuged at 750g for 10 minutes. Plasma was stored at −80°C with butylated hydroxytoluene (10 µl of 100 mM to each 1.5 ml eppendorf tube) to prevent artefactual oxidation. The complete methodologies for plasma total F2-isoprostanes, protein carbonyls, TAC, and GPX, including laboratory coefficients of variation (%CV), hve been previously reported by our group.Citation13 Patients were grouped by normal (≤250 pg/ml) or elevated (>250 pg/ml) oxidative stress based on plasma F2-isoprostanes. This was based on using a value 1.5 standard deviations (SDs) from mean values obtained from a previous study on apparently healthy 18–30-year old males and females from our laboratory.Citation13
Inflammation markers interleukin-6 (IL-6), interleukin-1β, tumour necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) were measured by an electrochemiluminescence technique using Human Pro-inflammatory 4-plex Ultra-sensitive Kit (Meso Sector S 6000, Rockville, MD). The assays were performed according to the manufacturer's instructions with %CV less than 20% being considered acceptable, as previously described.Citation14
Additional blood measures of lipids, haemoglobin, phosphate, creatinine, C-reactive protein (CRP), albumin, glucose, and insulin were conducted using standard laboratory techniques. The eGFR was estimated using the modification of diet in renal disease-175 formula.Citation15 Insulin resistance was computed using the homeostatic model assessment of insulin resistance method.Citation16 Stage 3 CKD was defined as eGFR 30 < 60 and stage 4 was defined as ≤29 ml/min/1.73 m2. Due to the small inclusion range of ≥25≤29 for stage 4 in this study, the majority of patients were classified as stage-3 CKD. Fasting blood samples were performed prior to any other testing. Beta blocker medication was withheld at least 12 hours prior to testing.
Grip strength was measured using a hand grip dynamometer (Jamar 5030 J1, Bolingbrook, IL, USA). Each participant received the same instructions after a demonstration by the tester, and the hand grip was adjusted accordingly for the patients comfort. Participants undertook the test six times, alternating between each hand. The maximum grip strength was attained from the highest reading of either hand. Age-predicted grip strength values were calculated by a predictive equation for males and females as reported by Desrosiers et al. (1995).Citation17 Cardiorespiratory fitness was measured as peak oxygen uptake (VO2peak) using expired air analysis (Vmax29c, SensorMedics, Yorba Linda, CA, USA) using the peak 20-second average of the final minute during a maximal treadmill test. The test protocol was determined by the Duke Activity Status Index, which was completed by the participants.Citation18 Based on participant's responses to this questionnaire, they performed either the Bruce, Balke, or Naughton protocols. Self-reported physical activity during the previous 6 months was determined using items from the Active Australia questionnaire.Citation19
Dual energy X-ray absorptiometry (DEXA), using whole body composition analysis, was used to assess lean mass (Hologic QDR 4500A version 12.6, Bedord, MA, USA). Lean mass percentage was calculated from the percentage of lean mass from the total mass. Appendicular lean mass percentage was calculated from the average of the four limbs. DEXA was performed on a representative sub-set of patients due to limited machine availability (n = 75).
Statistics
Mean ± SD was used to describe baseline characteristics, with percentages used to describe frequencies for categorical variables. A one-way analysis of variance with a Bonferroni post-hoc analysis was used to determine between group differences of grip strength tertiles. Univariate associations between variables and grip strength were evaluated using Pearsons correlations. Not normally distributed variables were transformed using the natural logarithm. Spearmans Rho was used for not normally distributed variables that were not able to be transformed. Significant univariate associations were included in a multivariate model to identify independent correlates, using the enter method. Regression diagnostics were assessed for identification of collinearity and variance inflation factor issues. The power analysis (pnorm using R statistical program) was performed using the sample size and testing for a single correlation coefficient between F2-isoprostanes and grip strength. Statistical analysis was performed using the IBM SPSS statistics 21 (New York, 2012). Statistical significance was assumed at P < 0.05.
Results
One hundred and fifty two patients were recruited and eligible for study participation between March 2008 and February 2013. shows the demographic and clinical data for this cohort. The mean age of patients was 60 years, the majority were obese and had low fitness. Nineteen patients were classified as stage-4 CKD and 141 patients were classified as stage-3 CKD. Sixty-eight (43.9%) patients had diabetes. There was no significant difference in F2-isoprostanes between patients with stage-3 and -4 CKD (P = 0.91) and patients with and without diabetes (P = 0.75). Thirty four patients (22.4%) had elevated F2-isoprostanes (≥250 pg/ml). There was no consequent increase in anti-oxidant status in patients with elevated F2-isoprostanes (GPX= normal F2-isoprostanes group 24.8 ± 2.9 (U/l) vs. elevated F2-isoprostanes group 19.6 ± 0.4, P = 0.6; TAC=normal F2-isoprostanes group 1.6 ± 0.1 (mmol/l) vs. elevated F2-isoprostanes group 1.8 ± 0.1, P = 0.2). There was also no significant correlation between F2-isoprostanes, protein carbonyls, GPX, and TAC (). There was, however, a moderate correlation between GPX and TAC (r = −0.245, P = 0.003).
Table 1. Patient characteristics
Table 2. Muscle atrophy variables and univariate relationship with F2-isoprostanes
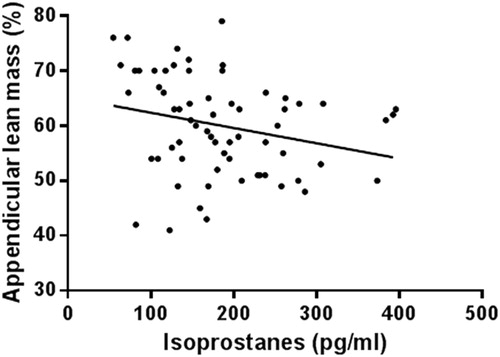
The association between F2-isoprostanes and other muscle atrophy markers are represented in . There was a negative association between F2-isoprostanes and grip strength (), and F2-isoprostanes and appendicular lean mass percentage (). There was no significant relationship between F2-isoprostanes and grip strength in patients with normal F2-isoprostanes (r = −0.161, P = 0.102). There were, however, significant associations between F2-isoprostanes and sex, albumin, Homeostatic model assessment-insulin resistance (HOMA-IR), haemoglobin, and BMI. There were no associations (P > 0.05) with inflammation markers IFN-γ, TNF-α, IL-6, and CRP. The power of detecting a significant association between grip strength and F2-isoprostanes in 152 participants is 99.3%. The correlation between grip strength and F2-isoprostanes in patients with BMI <30 kg/m2 (n = 41) was statistically significant (r = −0.353, P = 0.032). Despite the significant difference in F2-isoprostanes between males and females (males = 177 ± 89.1 pg/ml vs. females = 214.4 ± 99.1, P < 0.01), the correlation between grip strength and F2-isoprostanes in males was only approaching significance (r = −0.211, P = 0.067). Likewise, the correlation between grip strength and F2-isoprostanes in females was only not significantly correlated (r = −0.039, P = 0.772).
Figure 2. Association between F2-isoprostanes and appendicular lean mass percentage. r = −0.243, P = 0.04.
Grip strength was separated into relative tertiles of low (≤25 kg, n = 50), moderate (26–35 kg, n = 43), and high strength (≥36 kg, n = 44) (). There was a significant group difference between grip strength tertiles (P = 0.005), with post-hoc analyses identifying the low-strength group to have significantly higher levels of plasma F2-isoprostanes than the high-strength group (P = 0.006). The difference between low strength and moderate strength was approaching significance (P = 0.059). It was identified that 82% of all patients had grip strength values below their age-predicted grip strength (P < 0.001). Factors associated with grip strength are shown in . There was a strong correlation between grip strength and appendicular lean mass. Multiple linear regression found F2-isoprostanes to be a predictor of grip strength (β = −0.219, P = 0.032) independent of other predictors: sex, diabetes status, BMI, body fat percentage, and phosphate in a model also including age, IFN-γ, appendicular lean mass, haemoglobin, and VO2peak (adjusted r2 = 69.5, P < 0.001).
Figure 3. Plasma F2-isoprostane levels at low, moderate, and high tertiles of grip strength. Between group differences (P < 0.01). High grip strength has significantly lower plasma F2-isoprostanes than low grip strength (P < 0.01).
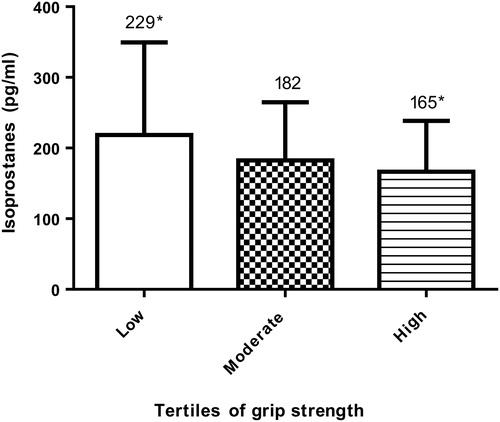
Table 3. Factors associated with grip strength
Discussion
This is the first study to investigate the role of oxidative stress in reduced lean mass and grip strength in CKD patients. Our main findings were: (1) 82% of CKD patients had grip strength lower than age-predicted normative values, (2) 22.4% of CKD patients had elevated F2-isoprostanes, (3) oxidative stress was negatively associated with variables associated with muscle atrophy, in particular, appendicular lean mass, grip strength, sex, age, albumin, HOMA-IR, haemoglobin, and BMI, and (4) plasma F2-isoprostanes were an independent predictor of reduced grip strength.
CKD results in significantly reduced muscle mass and strength.Citation20 Indeed, it was found that 82% of the CKD patients had grip strength lower than age-predicted normative values. It is likely that reduced strength is occurring at least partly as a consequence of muscle atrophy, evident by the strong positive correlation between grip strength and lean mass. Our findings are in support of the literature which identifies reduced strength in CKD patients, as a study on haemodialysis patients also found reduced strength to occur as a consequence of reduced muscle mass.Citation21 This is clinically relevant as reduced functional strength is closely associated with survival and low muscle mass is a potentially modifiable factor.Citation22 The almost linear relationship that occurs between grip strength and lean mass supports the use of grip strength as an inexpensive and easily used test in the field.
F2-isoprostanes were elevated in a significant proportion of patients; however, there was no subsequent increase in anti-oxidant status. In addition, there was no association between GPX, TAC, and F2-isoprostanes. These findings are supported by Karamouzis et al.Citation23 who found that as the stage of kidney disease increased, so too did the level of plasma F2-isoprostanes. This study also found that TAC did not change with advancing CKD stages. The disconnection between F2-isoprostanes and GPX and TAC in our findings suggests that an increase in oxidants is occurring without a compensatory increase in anti-oxidants.Citation23 This imbalance may be an important finding in elucidating the pathogenesis of oxidative injury in CKD patients. This is consistent with the findings from Dalla Libera et al.Citation24 who found an increase in muscle protein carbonylation and a blunted expression of stress proteins involved in the anti-oxidant defence in patients with disuse muscle dystrophy. As suggested by the authors of this study, future investigations are needed to identify the mechanisms responsible for the switching-off of these anti-oxidant defences, and whether challenging this mechanism can ameliorate muscle atrophy.Citation24
Plasma F2-isoprostane levels were identified to be negatively associated with both lean mass and grip strength. This finding suggests a relationship between oxidative stress and resultant loss of strength through reduced muscle mass, which has not previously been identified in CKD patients. It has previously been reported in haemodialysis patients that inflammatory markers are associated with muscle mass.Citation25 However in the current study, we were not able to demonstrate an independent association between inflammatory cytokines and grip strength or lean mass. It is possible that in less severe CKD patients (i.e. stage 3–4) oxidative stress may have a more significant contribution to muscle wasting than inflammation.
The significant association of F2-isoprostanes and grip strength in patients with BMI < 30 kg/m2 (n = 41) suggests that F2-isoprostanes is not just a marker of muscle function in obese patients. However, future studies should identify whether this relationship still exists in CKD patients with a BMI of <25 kg/m2. Sex and F2-isoprostanes were strongly correlated in all patients, and yet when separated into males and females the correlation between grip strength and F2-isoprostanes was no longer significant. This may indicate that a larger sample size is needed to detect significant correlations due to the high variability in F2-isoprostanes. Prior studies have reported inconsistent findings on sex differences and oxidative stress levels.Citation26–Citation29 We have shown that female CKD patients have higher levels of F2-isoprostanes than males. Therefore, future large-scale studies should identify whether the correlations between oxidative stress, lean mass, and strength is dependent on sex and whether changes in muscle parameters after an intervention differs between males and females.
Oxidative stress was associated with other variables related to muscle atrophy: hypoalbuminemia, increased insulin resistance, anaemia, and obesity. These factors have previously been proposed as variables associated with muscle atrophy.Citation30 It has been reported that increased reactive oxidative species (ROS) bind specific muscle proteins and increases the degradation by the Ubiquitin–Proteasome System.Citation31 This increase in degradation results in muscle atrophy.Citation32 In genetic muscle diseases, oxidative stress is implicated as the initiator and driver of muscle damage.Citation33
An obvious approach to the problem of reduced strength in CKD is exercise training. This potentially has a dual effect of not only strengthening muscle, but also correcting the imbalance between oxidative stress and protective anti-oxidant capacity. As exercise increases oxygen uptake, there is an acute increase in ROS production through the electron transport system.Citation34 With repeated exercise, anti-oxidant enzymes that combat this exercise-induced oxidative stress are upregulated to provide more protection against ROS.Citation35 Indeed, an investigation in exercise-induced malondialdehyde in rats also found a compensatory increase in GPX and superoxide dismutase.Citation36 Therefore, although exercise can cause acute increases in ROS, long-term exercise can prove to be a beneficial treatment in improving oxidative stress.Citation37 The randomized control trial following on from this baseline analysis involves an exercise intervention for 36 months and the impact of exercise training on oxidative stress will be examined. The current evidence on the protective effects of anti-oxidant therapies in improving kidney function remains equivocal.Citation38 However, using anti-oxidant therapy to attenuate ROS and increase muscle mass in the renal population has not previously been studied.Citation4
Limitations
The analysis from this study employs a cross-sectional design and therefore causality between oxidative stress, reduced grip strength, and lean mass cannot be determined. Despite the well-recognized validity of grip strength as an indication of overall upper body strength,Citation10 additional measures of strength, such as 1-repetition maximum testing, may have reinforced the findings. It should be noted that while DEXA provides an estimate of lean body mass, skeletal muscle histology, or magnetic resonance imaging to assess fibre and muscle size, would have provided a better measure of muscle atrophy. Despite this limitation, the associations found with lean mass are encouraging, as DEXA has been reported to underestimate the loss of thigh muscle mass in comparison to MRI.Citation39 Further work looking at the expression of other factors involved in muscle loss, such as atrogin-1 and myostatin in muscle biopsies of patients with CKD, is required to better understand the role of oxidative stress in the pathogenesis of muscle atrophy.
Conclusions
CKD patients have below average grip strength when compared to age-predicted normative values. It was identified that plasma F2-isoprostanes were independently associated with reduced strength in CKD patients. The findings from this study may assist in ascertaining appropriately targeted treatments for muscle loss, such as resistance exercises to restore muscle strength and long-term exercise training to correct the oxidative stress imbalance.
Disclaimer statements
Contributors KB conceived and designed the study, collected the data, analysed the data, interpreted the data and wrote the article in whole. EH collected the data, analysed the data, interpreting the data, revising the article. DS analysed the data and revised the article. DB analysed the data and revised the article. MR analysed the data and revised the article. NI obtained funding and ethics approval, interpreted the data and revised the article. JC conceived and designed the study, obtained funding and ethics approval, interpreted the data, wrote the article in part and revised the article.
Funding None.
Conflicts of interest None.
Ethics approval The study protocol was approved by the Princess Alexandra Human Research Ethics Committee (HREC 2007/190), and was registered at http://www.anzctr.org.au (Registration Number ANZCTR12608000337370). All patients gave written, informed consent to participate in this study.
Acknowledgements
The authors of this paper would like to acknowledge Dr David Vesey from Princess Alexandra Hospital Pathology for his work in analysing the inflammation measures. The funding support provided by the NHMRC-funded Centre for Clinical Research Excellence – Vascular and Metabolic Health (CCRE), University of Queensland (UQ) and the Department of Nephrology, Princess Alexandra Hospital is acknowledged. Work supported by the NRMRC Australia, through Australia Fellowship award #511081, CKD.QLD (Chronic Kidney Disease in Queensland) and Centre for Chronic Disease at the University of Queensland.
References
- Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 2010;91(4):1128–32.
- Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 2008;27(4):557–64.
- Jones DP. Redefining oxidative stress. Antiox Redox Signal 2006;8(9–10):1865–79.
- Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 2012;17(4):311–21.
- Yin H. New techniques to detect oxidative stress markers: mass spectrometry-based methods to detect isoprostanes as the gold standard for oxidative stress in vivo. Biofactors 2008;34(2):109–24.
- Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 2004;65(3):1009–16.
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 2002;62(5):1524–38.
- Himmelfarb J. Relevance of oxidative pathways in the pathophysiology of chronic kidney disease. Cardiol Clin 2005;23(3):319–30.
- Crowe AV, McArdle A, McArdle F, Pattwell DM, Bell GM, Kemp GJ, et al. Markers of oxidative stress in the skeletal muscle of patients on haemodialysis. Nephrol Dial Transplant 2007;22(4):1177–83.
- Bohannon RW. Hand-grip dynamometry provides a valid indication of upper extremity strength impairment in home care patients. J Hand Ther 1998;11(4):258–60.
- Newman AB, Kupelian V, Visser M, Simonsick EM. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol 2006;61(1):72–7
- Heimbürger O, Qureshi AR, Blaner WS, Berglund L, Stenvinkel P. Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am J Kidney Dis 2000;36(6):1213–25.
- Mullins AL, van Rosendal SP, Briskey DR, Fassett RG, Wilson GR, Coombes JS. Variability in oxidative stress biomarkers following a maximal exercise test. Biomarkers 2013;18(5):446–54.
- Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex® and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods 2009;340(1):55–64.
- Levey AS, Coresh J, Greene T, Stevens LA, Yaping Z, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145(4):247–61.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–9.
- Desrosiers J, Bravo G, Hébert R, Dutil E. Normative data for grip strength of elderly men and women. Am J Occup Ther 1995;49(7):637–644.
- Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989;64(10):651–4.
- Heesch KC, Hill RL, van Uffelen JGZ, Brown WJ. Are Active Australia physical activity questions valid for older adults? J Sci Med Sport 2011;14(3):233–7.
- Wang XH, Du J, Klein JD, Bailey JL, Mitch WE. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int 2009;76(7):751–9.
- Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent-Braun JA. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int 2003;63(1):291–7.
- Yoda M, Inaba M, Okuno S, Yoda K, Yamada S, Imanishi Y, et al. Poor muscle quality as a predictor of high mortality independent of diabetes in hemodialysis patients. Biomed Pharmacother 2012;66(4):266–70.
- Karamouzis I, Sarafidis PA, Karamouzis M, Iliadis S, Haidich AB, Sioulis A, et al. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol 2008;28(3):397–404.
- Dalla Libera L, Ravara B, Gobbo V, Tarricone E, Vitadello M, Biolo G, et al. A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J Appl Physiol 2009;107(2):549–57.
- Kaizu Y, Ohkawa S, Odamaki M, Ikegaya N, Hibi I, Miyaji K, et al. Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis 2003;42(2):295–302.
- Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, et al. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol 2002;22(3):438–42.
- Bloomer RJ, Fisher-Wellman KH. Blood oxidative stress biomarkers: influence of sex, exercise training status, and dietary intake. Gend Med 2008;5(3):218–28.
- Brunelli E, Domanico F, La Russa D, Pellegrino D. Sex differences in oxidative stress biomarkers. Curr Drug Targets 2014;15(8):811–5.
- Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol 2007;34(9):938–45.
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27(6):793–9.
- Livnat-Levanon N, Glickman MH. Ubiquitin–Proteasome System and mitochondria – reciprocity. Biochim Biophys Acta – Gene Regul Mech 2011;1809(2):80–7.
- Heo J-M, Rutter J. Ubiquitin-dependent mitochondrial protein degradation. Int J Biochem Cell Biol 2011;43(10):1422–6.
- Haycock JW, MacNeil S, Jones P, Harris JB, Mantle D. Oxidative damage to muscle protein in Duchenne muscular dystrophy. Neuroreport 1996;8(1):357–61.
- Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann NY Acad Sci 2001;928(1):236–47.
- Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med 2011;51(5):942–50.
- Lawler JM, Powers SK, Visser T, Dijk HV, Kordus MJ, Ji LL. Acute exercise and skeletal muscle antioxidant and metabolic enzymes: effects of fiber type and age. Am J Physiol Regul Integr Comp Physiol 1993;265(6):1344–50.
- Miyazaki H, Oh-ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S, et al. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol 2001;84(1–2):1–6.
- Coombes JS, Fassett RG. Antioxidant therapy in hemodialysis patients: a systematic review. Kidney Int 2012;81(3):233–46.
- Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact 2013;13(3):320–8.