Abstract
Objectives: We investigated whether pomegranate extract plays a protective antioxidant role against mesenteric ischemia–reperfusion injury (IR), which can lead to a systemic response and damage distant organs, such as the lung, liver, and kidney.
Methods: Forty female Wistar–Albino rats were separated into four groups: laparotomy, laparotomy + PG, mesenteric IR, and mesenteric IR and pomegranate (IR + PG). In the laparotomy + PG and IR + PG groups, pomegranate (225 mg/kg) was given by oral gavage at the beginning of the study. Ischemia was induced for 30 minutes, and reperfusion was subsequently allowed for 60 minutes in the IR and IR + PG groups. The malondialdehyde (MDA) and total antioxidant activity (AOA) levels were evaluated in blood samples. Additionally, all tissues were removed for the measurement of AOA and total oxidant status as well as for subsequent histopathological evaluation. The oxidative stress index was calculated.
Results: Histopathological changes in all organs were significantly higher in the IR group and significantly lower in the IR + PG group vs. the other groups. Serum MDA levels were significantly lower in the IR + PG group than in the IR group. No significant difference was found in AOA levels of the groups.
Discussion: These data may explain the positive protective effects of pomegranate based on the histopathologic findings in ischemic conditions in an intestinal IR injury model.
Introduction
Ischemia-associated diseases are among the most important causes of morbidity and mortality. Acute intestinal ischemic injury is caused by a decrease in blood flow (due to, e.g., intussusception, incarcerated hernia, necrotizing enterocolitis, transplantation, shock, and sepsis), leading to tissue damage. This tissue damage triggers mechanisms of cellular damage, including microvascular permeability, interstitial edema, damaged vasoregulation, and inflammatory cell infiltration.Citation1 Despite the known primary cellular damage, the pathophysiology of intestinal ischemic injury cannot be described without mentioning the mechanisms involved in reperfusion injury. Some reperfusion mechanisms are uncertain, while others are now well documented. For example, oxidative stress mediators, such as reactive oxygen species (ROS), polymorphonuclear neutrophil activation, and nitric oxide production, are known to be major determinants of reperfusion injury. Many cellular models of the mechanisms of cellular dysfunction in ischemia–reperfusion (IR) injury are available.Citation2–Citation4
Defense against oxidative stress is mediated by antioxidant enzymes (e.g. superoxide dismutase, catalase, and glutathione peroxidase), which process and remove free radicals and other reactive products. Other mechanisms are non-enzymatic and include many natural or synthetic antioxidants (e.g. glutathione and alpha-tocopherol) that prevent oxidative damage by reacting with free radicals. A diet containing low-molecular-weight antioxidants benefits antioxidant defense.Citation5,Citation6
The rich phenolic contents of pomegranate include anthocyanins and hydrolyzable tannins.Citation7,Citation8 Maternal dietary supplementation with pomegranate juice is neuroprotective against hypoxic–ischemic injury of the neonatal mouse brain.Citation9 Pomegranate juice also has protective effects against systemic oxidative stress in mice.Citation10 Investigations have shown that pomegranate extract has gastroprotective activity through antioxidant mechanisms.Citation11 Using pomegranate extract as a dietary supplement has been shown to ameliorate colonic inflammation. A review of these studies indicates that pomegranate exerts beneficial effects on oxidative stress and inflammation.Citation12
Mesenteric IR injury and its effects on distant organs are closely linked to oxidative stress and inflammation. Thus, we sought to investigate the potential protective antioxidant effects of the pomegranate (Punica granatum) in a mesenteric IR model.
Materials and methods
Animal subjects
In this study, 40 female Wistar–Albino rats, weighing 200–250g, were obtained from the BLIND University School of Medicine Animal Care. Simple randomization method was used for selection of the animals in the groups. The Local Ethics Committee approved the study. All experimental protocols were performed according to the guidelines for the ethical treatment of experimental animals.
Rats were housed in an air-conditioned room with a 12/12-hour light/dark cycle and a constant temperature (22 ± 2°C). The rats were housed in cages and provided standard rat chow and water ad libitum before the experiments. The animals were fasted overnight before the experiments but were given free access to water. Rats were anesthetized with 50 mg/kg ketamine hydrochloride (Ketalar, Parke Davis, Istanbul, Turkey) and 10 mg/kg xylazine (Rompun, Bayer AG, Leverkusen, Germany) intramuscularly prior to the surgical procedures.
Study protocol
After the abdomen was shaved and disinfected, a midline incision was made and the rats underwent either laparotomy surgery or IR. Ischemia was induced by clamping the superior mesenteric artery and collateral vessels with an atraumatic vascular clamp at its origin for 30 minutes. Mesenteric ischemia was confirmed by the loss of mesenteric pulsations and the blanching of the intestine. During the surgical procedures, the peritoneal cavity was filled with warm Ringer's lactate to prevent hypovolemia and hypothermia during the ischemic episode, the abdominal incision was closed temporarily. Then, 30 minutes later, the ischemic intestine was reperfused by removing the clamp for 60 minutes. Reperfusion was confirmed by the restoration of pulsation and color prior to closing the incision. At the end of this period, the animals were sacrificed by removing blood from the heart.
The animals were divided into four groups. In Group 1 (laparotomy), only laparotomy was performed. In Group 2 (laparotomy + PG), laparotomy was performed, and the animals were given 225 mg/kg pomegranate (transport maximum (Tmax): 30 minutes; Pomella, Verdure Sciences, Noblesville, IN, USA)Citation13 by oral gavage at the beginning of the experiment. In Group 3 (IR), the superior mesenteric artery and collateral vessels were clamped. After 30 minutes, reperfusion was allowed for 60 minutes. In Group 4 (IR + PG), the superior mesenteric artery and collateral vessels were clamped. After 30 minutes, reperfusion was allowed for 60 minutes, and the rats were given 225 mg/kg pomegranate (Tmax: 30 minutes; Pomella) by oral gavage at the beginning of the experiment.
At the end of the procedures, blood and tissue samples from the intestine, liver, lung, and kidney were obtained for biochemical and histopathological analysis. The intestine, liver, kidney, and lung tissues were divided for histopathological examination by removing adequate sample amounts and transferring the excess tissue to plastic clam-shell containers containing 10% formaldehyde solution for either further histological evaluation or storage at 80°C for the subsequent determination of the antioxidant activity (AOA), total oxidant status (TOA), and oxidative stress index (OSI) levels.
Biochemical analyses
Blood sampling
Venous blood samples were collected in tubes with a gel separator and centrifuged for 5 minutes at 1550g. The supernatant plasma was removed and placed in polypropylene plastic tubes. The tubes were properly labeled with the appropriate sample name and type. Samples were taken and stored at −80°C for the determination of the TOA, AOA, and malondialdehyde (MDA).
Tissue homogenization
To estimate the tissue oxidant and antioxidant levels, tissue samples were prepared at 4°C. The tissues were weighed, cut into small pieces, and homogenized in 10 volumes of ice-cold phosphate buffer (50 mM, pH 7.0) using a glass Teflon homogenizer (Ultra-Turrax T8, Staufen, Germany). The resulting homogenate was centrifuged (26 000g, 10 minutes, 4°C), and the supernatant was used immediately for the determination of the TOA and AOA.
Measurement of MDA
The MDA levels were estimated using the double-heating method of Draper et al.Citation14 This method is based on spectrophotometric measurements of the color generated by the reaction of thiobarbituric acid (TBA) and MDA. For this purpose, 2.5 ml of trichloroacetic acid solution (10%) was added to 0.5 ml plasma in a centrifuge tube, and the tubes were placed in boiling water for 15 minutes. After cooling in tap water, the tubes were centrifuged (1000g, 10 minutes), and 2 ml of the supernatant was added to 1 ml of the TBA solution (6.7 g/l) in a test tube. The tube was then placed in boiling water for 15 minutes. The solution was cooled in tap water, and its absorbance at 532 nm was measured using a spectrophotometer (Shimadzu UV-1208, Japan). The MDA concentration was calculated from the absorbance coefficient of the MDA–TBA complex (an absorbance coefficient of 1.56 × 105/(cm M)) and expressed as µmol/l. All MDA analytical steps were performed in the Biochemistry Department Laboratory (BLIND University Medical Faculty).
Measurement of TOA
The TOA of the supernatant fractions was determined using a novel automated measurement method, developed by Erel.Citation15 Oxidants present in the sample oxidize the ferrous iron–o-dianisidine complex to ferric ions. The oxidation reaction is enhanced by glycerol molecules, which are abundant in the reaction medium. The ferric ion produces a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay was calibrated with hydrogen peroxide, and the results are expressed in the nmol H2O2 equiv./mg protein.
Measurement of AOA
The AOA of the supernatant fractions was determined using a novel automated measurement method developed by Erel.Citation16 In this method, the hydroxyl radical, the most potent biological radical, can be produced. In the assay, the ferrous ion solution present in Reagent 1 is mixed with the hydrogen peroxide in Reagent 2. The sequentially produced radicals, such as the brown-colored dianisidinyl radical cation produced by the hydroxyl radical, are also potent radicals. Using this method, the antioxidative effects of the sample against free radical reactions initiated by the produced hydroxyl radical can be measured. The assay has an excellent precision of less than 3%. The results are expressed as nmol Trolox equiv./mg protein.
Oxidative stress index
The OSI is the ratio of total peroxide to total antioxidant potential, an indicator of the degree of oxidative stress. It was calculated as follows:Citation17
Histopathological evaluation
Intestine, liver, kidney, and lung tissues were scored from mild to severe injury. Tissue specimens were fixed in 10% formalin for 24 hours, then embedded in paraffin wax, and cut into 4-μm sections. Slides were stained with hematoxylin and eosin (H&E) and examined under a light microscope using a standard protocol. Each slide was evaluated by a pathologist who was blinded to the grouping and treatment of the specimen under analysis.
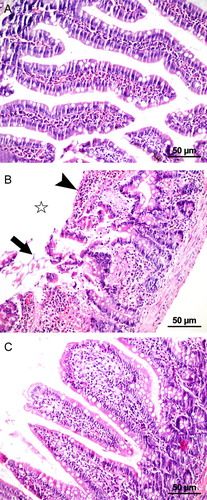
Intestinal injury was evaluated on a scale from 0 to 5 points. The tissue was assessed as having no diagnostic change, increased lamina propria cellularity, congestion and expansion of the subepithelial capillary, capillary dilatation, villus fragmentation, disintegration of the lamina propria with ulceration, and hemorrhageCitation18 (Fig. ).
Figure 1 Microscopic images of the jejunum showing the histopathological samples of laparotomy (A), IR (B), and IR + PG (C) groups (H&E, 200×). (A) Mucosa with normal villous formation. (B) Disintegration of the villous (star), capillary dilatation, ulceration of lamina propria (arrow), capillary dilatation, infiltration, and hemorrhage (arrowhead) after IR injury. (C) Restitution of jejunal mucosa in the pomegranate-applied group.
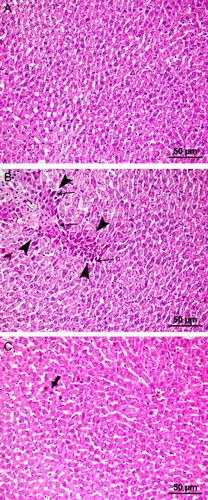
Hepatic injury was evaluated according to its severity using the following ordinal scale: grade 0, minimal or no evidence of injury; grade 1, mild injury with cytoplasmic vacuolation and focal nuclear pyknosis; grade 2, moderate-to-severe injury with enlarged nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; and grade 3, severe necrosis with disintegration of hepatic cords, hemorrhage, and neutrophil infiltration (Fig. ).
Figure 2 Microscopic images of the liver showing the histopathological samples of laparotomy (A), IR (B), and IR + PG (C) groups (H&E, 200×). (A) Normal hepatocyte formation. (B) Degenerative changes (area marked with arrowheads); necrosis and picnotic nucleus (arrows). (C) Hepatocytes in pomegranate applied group with few picnotic changes (arrows).
Renal injury was graded as follows: grade 0, no visible change; grade 1, tubular cell swelling, brush border loss, and nuclear condensation with up to one-third of the tubular profile showing nuclear loss; grade 2, the same as grade 1 but with greater than one-third and less than two-thirds of the tubular profile showing nuclear loss; and grade 3, with greater than two-thirds of the tubular profile showing nuclear loss.
Lung injury was evaluated using the following ordinal scale: grade 0, no change; grade 1, mild neutrophil leukocyte infiltration and mild-to-moderate interstitial congestion; grade 2, moderate neutrophil leukocyte infiltration, perivascular edema formation, and disintegration of structure; and grade 3, dense neutrophil leukocyte infiltration and the destruction of pulmonary structures.Citation19
Statistical analysis
SPSS software (ver. 11.5 for Windows; SPSS, Inc., Chicago, IL, USA) was used for the statistical analyses. All values are given as the means ± standard error of mean (SEM). Differences between the groups were evaluated by Kruskal–Wallis variance analysis followed by a Mann–Whitney U test with a Bonferroni correction for binary comparisons. Spearman's test was used to determine the correlations between the groups. P-values <0.05 were considered to indicate statistical significance.
Results
The oxidative and antioxidative parameters of all groups are given in Table . The levels of MDA, a marker of oxidative stress, were significantly higher in the IR group than in the laparotomy group (P = 0.003). There were no significant differences between the MDA levels in the IR + PG and laparotomy groups. MDA levels were significantly higher in the IR group compared with the IR + PG group. AOA levels in the IR and IR + PG groups were significantly higher than in the laparotomy group (P = 0.005, P = 0.019, respectively). There was no significant difference in AOA levels between the IR and IR + PG groups.
Table 1 Oxidative and antioxidative parameters of all groups
No significant difference was found between the IR and IR + PG groups in terms of the AOA and TOA values in kidney and lung tissue. However, liver AOA values were significantly higher in the IR + PG group than in the IR group.
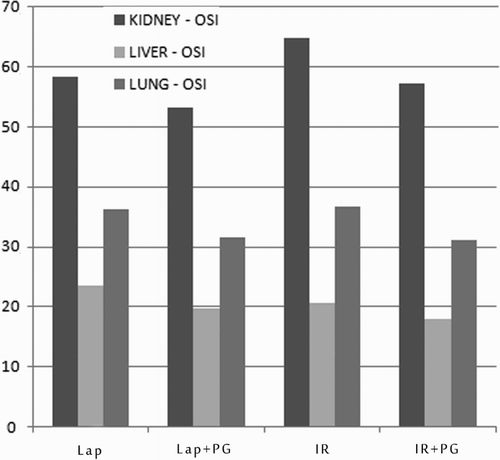
TOA levels did not differ significantly between the IR and IR + PG groups. Liver, lung, and kidney OSI levels were significantly higher in the IR group than in the IR + PG group. Furthermore, the lung and liver OSI levels were significantly higher in the laparotomy group than in the laparotomy + PG group (Fig. ).
Figure 3 Comparison of OSI levels in the groups. Lap, laparotomy; PG, pomegranate; IR, ischemia–reperfusion.
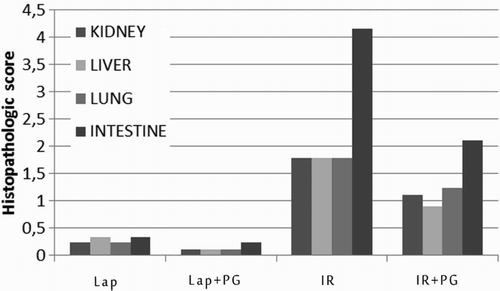
Histopathological scores in all organs were significantly higher in the IR group and significantly lower in the IR + PG group vs. the other groups (Fig. ). The intestinal histopathological scores were well correlated with MDA levels (P < 0.001, r = 0.566), kidney histopathological score (P < 0.001, r = 0.789), liver histopathological score (P < 0.001, r = 0.659), and lung histopathological score (P < 0.001, r = 0.749).
Discussion
IR injury caused by the temporary decrease in blood flow in an organ leads to tissue damage and loss of function. Our study showed that the IR-induced oxidative stress indices and cellular disintegration of local and distant organs, in particular, were decreased by pomegranate extract administration before IR protocol. The histopathological scores of the IR group were significantly higher than IR + PE group (P < 0.001) in local and all distant organs.
Hypoxia and reoxygenation are the major causes of IR injury. Many studies have shown that reoxygenation is more responsible for the mechanisms of IR injury than the hypoxia itself.Citation1,Citation20 It is recognized that pathological process of IR injury principally stresses the systemic inflammatory response syndrome and may lead to the multiorgan dysfunction. In that manner, IR injury affects multiple organs in specific ways. Many pathophysiological mechanisms of ischemic tissue injury have been suggested. Recent studies of tissue damage have focused on leukocytes and leukocyte adhesion molecules as well as their effects of oxygen free radical production,Citation21 cytotoxic enzyme release,Citation22 and increased cytokine release.Citation23 Emerging hydroxyl radicals react with membrane structures, causing lipid peroxidation. Lipid peroxidation leads to cell damage, which can cause cell death.Citation24
Defense against oxidative reactants is mediated by antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase. Additionally, many native molecules, such as glutathione, vitamin E (alpha-tocopherol), and ascorbic acid, exert antioxidant effects.Citation25,Citation26 Thus, studies of IR injury have focused on potential antioxidants that are natural components of various food items which are in daily use.Citation27,Citation28 Fruits and vegetables have high antioxidant activities, depending on their phenolic contents, which remove free radicals.Citation29 Among them, the pomegranate has a high phenolic content, containing anthocyanins, tannin, and ellagitannin, and is known to have marked antioxidant and anti-inflammatory effects.Citation30 A comparative study has shown that anthocyanin has a greater antioxidant effect than vitamin E (alpha-tocopherol), ascorbic acid, or beta-carotene.Citation31
Oxidative stress causes lipid peroxidation. MDA is an end product of lipid peroxidation and is a well-known indicator of free radical formation in intestinal tissues.Citation32 In this study, not surprisingly, we found that the MDA levels were significantly higher in the IR group (Group 3) than in the laparotomy group (Group 4) (P = 0.003). Pomegranate is suggested to decrease MDA levels.Citation33 Similarly, when evaluating the preventative effects of pomegranate against lipid peroxidation, we found that MDA levels were significantly lower in the IR + PG group (Group 3) than in the IR group (Group 4) (P = 0.031).
Lung injury plays a key role in intestinal IR injury. Blood flow from damaged post-ischemic tissues drains predominantly into the pulmonary blood pool. Blood derived from ischemic tissue is rich in neutrophils and macrophages; these cell types produce cytokines and trigger an inflammatory response, causing alveolar capillary endothelial cell injury and pulmonary edema.Citation34,Citation35 Pomegranate inhibits ROS-dependent luminol-amplified chemiluminescence in both resting and stimulated neutrophils and markedly inhibits myeloperoxidase activity, and it attenuates lipopolysaccharide-induced lung inflammation in mice.Citation36 Similarly, in the present study, lung TOA (P = 0.049) and lung OSI levels (P = 0.034), which are oxidative stress parameters, were found to be significantly higher in the laparotomy group than in the laparotomy + PG group However, irrespective of the absence of ischemia in the laparotomy group, oxidants could be generated by the laparotomy; therefore, pomegranate decreased the laparotomy-induced oxidative stress values in both the laparotomy + PG and laparotomy groups. Lung OSI levels and lung histopathological scores were higher in the IR group than in the IR + PG group (P = 0.049) (Table ). Pre-treatment with pomegranate reduced the lung tissue histopathological findings (P = 0.019).
Table 2 Histopathologic evaluation of intestine, kidney, liver, and lung for each group
Intestinal IR injury causes the release of cytokines and pro-inflammatory mediators (such as oxygen free radicals, platelet-activating factor, arachidonic acid metabolites, IL-6, IL-8, ICAM-1, and TNF-alpha) from damaged mesenteric tissue. These pro-inflammatory mediators pass into the blood through the lymphatic pathway and act on polymorphonuclear leukocytes in remote organs, such as the liver and kidney, causing cytotoxic cellular effects and increasing the levels of ROS in the affected organs.Citation37,Citation38 In the present study, the intestinal histopathological scores were well correlated significantly with the liver (r = 659, P < 0.001) and kidney (r = 789, P < 0.001) histopathological scores (Table ). Pomegranate juice consumption was found to reduce the hepatic oxidative stress scores in mice,Citation17 and pomegranate seed extract was found to exert protective effects against cisplatin hepatotoxicity in rabbits.Citation39 In our study, liver histopathological scores were not significantly different between the laparotomy and laparotomy + PG groups (Table ). However, liver OSI levels were significantly higher in the laparotomy group (P = 0.041). Liver AOA levels were higher, and liver OSI and liver histopathological scores were significantly lower in the IR + PG group vs. the IR group. These results suggested that pomegranate decreases oxidative damage to the liver tissue.
Similar results in renal tissue have been reported in previous studies. In a recent study from Cekmen et al.,Citation33 pomegranate was reported to be preventive for oxidative stress in kidneys caused by gentamicin treatment. A different study demonstrated that pomegranate had a protective role against carbon tetrachloride-induced toxicity in the kidneys.Citation40 Another clinical study showed that pomegranate can attenuate hexachlorobutadiene-induced nephrotoxicity, with clinical findings of a dose-dependent decrease in serum creatinine and urea levels. In this study, as in previous reports, the kidney OSI levels and histopathological scores were significantly higher in the IR group compared with the IR + PG group (respectively, P = 0.049, P = 0.017) (Table ). It should be noted that our results on the kidneys were achieved using a distant IR protocol.Citation41
Unlike previous reports, this study found that the AOA levels were lower in the IR + PG group compared with the IR group. AOA levels increased in response to oxidative stress. If the oxidative effects were reduced, AOA levels would be expected to be lower than in the IR + PG group. In contrast, the bioavailability of pomegranate is low in animals following oral consumption. Furthermore, the ellagitannins, which have an antioxidant effect, are connected to cellular DNA and proteins, thus limiting the oral absorption of pomegranate.Citation42,Citation43
There were some limitations in our study. We reported only a single point of reperfusion because we had rat deaths; blood samples could not be supplied for more than 60 minutes in all groups. Additional groups for determining the time variation of pomegranate consumption and dose titration curve may be more helpful. Cytokine levels and quantitation of neutrophils in tissues were not studied. Female-only option might influence on the inflammatory markers because of hormonal changes.
In conclusion, the consumption of pomegranate extract can ameliorate the oxidative stress effect, particularly in terms of the degenerative histopathological changes in tissues damaged by IR injury. Pomegranate was also found to exert beneficial effects by preventing distant organ damage after IR injury. However, the protective effects of pomegranate must be supported by further studies.
Disclaimer statements
Contributors None.
Funding This study has not received financial support from any organization.
Conflicts of interest I (we) certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Ethics approval Dicle University School of Medicine Animal Care. The Local Ethics Committee approved the study.
References
- Granger DN, Korthuis RJ. Physiologic mechanisms of post ischemic tissue injury. Annu Rev Physiol 1995;57:311–32. doi: 10.1146/annurev.ph.57.030195.001523
- Sreejayan RMN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol 1994;46:1013–6. doi: 10.1111/j.2042-7158.1994.tb03258.x
- Karabulut AB, Kirimlioglu V, Kirimlioglu H, Yilmaz S, Isik B, Isikgil O. Protective effects of resveratrol on spleen and ileum in rats subjected to ischemia–reperfusion. Transplant Proc 2006;38:375–7. doi: 10.1016/j.transproceed.2006.01.017
- Mallick IH, Yang WX, Winslet MC, Seifalian AM. Pyrrolidine dithiocarbamate reduces ischemia-reperfusion injury of the small intestine. World J Gastroenterol 2005;11:7308–13. doi: 10.3748/wjg.v11.i46.7308
- Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 2006;16:577–86. doi: 10.1016/j.jnutbio.2005.05.013
- Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent coordinately regulated defences against oxidative stress. Free Radic Res 1999;31:273–300. doi: 10.1080/10715769900300851
- Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal 2008;10:475–510. doi: 10.1089/ars.2007.1740
- Narr Ben C, Ayed N, Metche M. Quantitative determination of the polyphenolic content of pomegranate peel. Z Lebensm Unters Forsch 1996;203:374–8. doi: 10.1007/BF01231077
- Loren DJ, Seeram NP, Schulman RN, Holtzman DM. Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic–ischemic brain injury. Pediatr Res 2005;57:858–64. doi: 10.1203/01.PDR.0000157722.07810.15
- Faria A, Monteiro R, Mateus N, Azevedo I, Calhau C. Effect of pomegranate juice intake on hepatic oxidative stress. Eur J Nutr 2007;46:271–8. doi: 10.1007/s00394-007-0661-z
- Alam MS, Alam MA, Ahmad S, Najmi AK, Asif M, Jahangir T. Protective effects of Punica granatum in experimentally-induced gastric ulcers. Toxicol Mech Methods 2010;20:572–8. doi: 10.3109/15376516.2010.508079
- Rosillo MA, Sánchez-Hidalgo M, Cárdeno A, Aparicio-Soto M, Sánchez-Fidalgo S, Villegas I et al. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol Res 2012;66:235–42. doi: 10.1016/j.phrs.2012.05.006
- Sancaktutar AA, Bodakci MN, Hatipoglu NK, Soylemez H, Basarılı K, Turkcu G. The protective effects of pomegranate extracts against renal ischemia–reperfusion injury in male rats. Urol Ann 2014;6:46–50. doi: 10.4103/0974-7796.127029
- Draper HH, Csallony AS, Hadley N. Urinary aldehydes as indicators of lipid peroxidation in vivo. Free Radic Biol Med 2000;29:1071–7. doi: 10.1016/S0891-5849(00)00367-1
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004;37:112–9. doi: 10.1016/j.clinbiochem.2003.10.014
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008
- Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis 2005;5:95. doi: 10.1186/1471-2334-5-95
- Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN et al. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 1970;101:478. doi: 10.1001/archsurg.1970.01340280030009
- Kesik V, Guven A, Vurucu S, Tunc T, Uysal B, Gundogdu G et al. Melatonin and 1400 W ameliorate both intestinal and remote organ injury following mesenteric ischemia/reperfusion. J Surg Res 2009;1:1–9.
- Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int 1993;43:1160–78. doi: 10.1038/ki.1993.163
- Murota S, Fujita H, Wakabayashi Y, Morita I. Cell adhesion molecule mediates endothelial cell injury caused by activated neutrophils. Keio J Med 1996;45:207–12. doi: 10.2302/kjm.45.207
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 1989;320:365–76. doi: 10.1056/NEJM198902093200606
- Chamoun F, Burne M, O'Donnell M, Rabb H. Pathophysiologic role of selectins and their ligands in ischemia reperfusion injury. Front Biosci 2000;5:103–9. doi: 10.2741/chamoun
- Girotti AW. Lipid hydroperoxide generation, turnover and effector action in biological systems. J Lipid Res 1998;39:1529–42.
- Scandalios JG. The rise of ROS. Trends Biochem Sci 2002;27:483–6. doi: 10.1016/S0968-0004(02)02170-9
- Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL et al. Oxygen radicals and human disease. Ann Intern Med 1987;107:526–45. doi: 10.7326/0003-4819-107-4-526
- Avci G, Kadioglu H, Sehirli AO, Bozkurt S, Guclu O, Arslan E et al. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J Surg Res 2012;172:39–46. doi: 10.1016/j.jss.2011.08.021
- Song X, Xu H, Feng Y, Li X, Lin M, Cao L. Protective effect of grape seed proanthocyanidins against liver ischemic–reperfusion injury: particularly in diet-induced obese mice. Int J Biol Sci 2012;8:1345–62. doi: 10.7150/ijbs.4699
- Heinonen IM, Meyer AS, Frankel EN. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J Agric Food Chem 1998;46:4107–12. doi: 10.1021/jf980181c
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 2000;48:4581–9. doi: 10.1021/jf000404a
- Seeram NP, Nair MG. Inhibition of lipid peroxidation and structure activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J Agric Food Chem 2002;50:5308–12. doi: 10.1021/jf025671q
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animals and tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351. doi: 10.1016/0003-2697(79)90738-3
- Cekmen M1, Otunctemur A, Ozbek E, Cakir SS, Dursun M, Polat EC et al. Pomegranate extract attenuates gentamicin-induced nephrotoxicity in rats by reducing oxidative stress. Ren Fail 2013;35:268–74. doi: 10.3109/0886022X.2012.743859
- Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. The role of the lung in postinjury multiple organ failure. Surgery 2005;138:749–57. doi: 10.1016/j.surg.2005.07.020
- Mura M1, Andrade CF, Han B, Seth R, Zhang Y, Bai XH et al. Intestinal ischemia–reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock 2007;28(2):227–38. doi: 10.1097/01.shk.0000278497.47041.e3
- Bachoual R, Talmoudi W, Boussetta T, Braut F, El-Benna J. An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem Toxicol 2011;49:1224–8. doi: 10.1016/j.fct.2011.02.024
- Senthil M, Brown M, Xu DZ. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma 2006;60:958–65. doi: 10.1097/01.ta.0000215500.00018.47
- Parks DA, Bulkley GB, Granger DN. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology 1982;82:9–15.
- Yildirim NC, Kandemir FM, Ceribasi S, Ozkaraca M, Benzer F. Pomegranate seed extract attenuates chemotherapy-induced liver damage in an experimental model of rabbits. Cell Mol Biol 2013;2:59.
- Abdel Moneim AE, El-Khadragy MF. The potential effects of pomegranate (Punica granatum) juice on carbon tetrachloride-induced nephrotoxicity in rats. J Physiol Biochem 2013;69:359–70. doi: 10.1007/s13105-012-0218-3
- Bouroshaki MT, Sadeghnia HR, Banihasan M, Yavari S. Protective effect of pomegranate seed oil on hexachlorobutadiene-induced nephrotoxicity in rat kidneys. Ren Fail 2010;32:612–7. doi: 10.3109/08860221003778056
- Clifford MN, Scalbert A. Ellagitannins – nature, occurrence and dietary burden. J Sci Food Agric 2000;80:1118–25. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1118::AID-JSFA570>3.0.CO;2-9
- Whitley AC, Stoner GD, Darby MV, Walle T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid-extensive binding to protein and DNA. Biochem Pharmacol 2003;66:907–15. doi: 10.1016/S0006-2952(03)00413-1