Abstract
Oxidative stress results from a prooxidant-antioxidant imbalance, leading to cellular damage. It is mediated by free radicals, such as reactive oxygen species or reactive nitrogen species, that are generated during physiological aerobic metabolism and pathological inflammatory processes. Skin serves as a protective organ that plays an important role in defending both external and internal toxic stimuli and maintaining homeostasis. It is becoming increasingly evident that oxidative stress is involved in numerous skin diseases and that antioxidative strategies can serve as effective and easy methods for improving these conditions. Herein, we review dysregulated antioxidant systems and antioxidative therapeutic strategies in dermatology.
Introduction
Reactive oxygen species (ROS) include superoxide anion , peroxides, hydroxyl radical (OH°, and singlet oxygen
.Citation1 These molecules activate proliferative and cell survival signaling and can damage DNA (DNA base damage, DNA single-strand and double-strand breaks, DNA and protein crosslinks, DNA and chromosomal aberration), lipid membranes, collagen structures, and mitochondrial function. ROS are produced by keratinocytes and virtually all types of skin cells in response to signals from cytokines, growth factors, airborne pollutants, UV radiation, food additives/preservatives, cosmetics, drugs, and physiologic stimuli. Antioxidants are generally classified as endogenous and exogenous. The skin has a vast antioxidant system, including enzymatic antioxidants, such as glutathione peroxidase (GPX), glutathione S-transferase, glutathione reductase, superoxide dismutase (SOD) and catalase, as well as non-enzymatic antioxidants, including ascorbic acid (vitamin C), glutathione (GSH), ubiquinol, uric acid, vitamin A, melanin, alpha-tocopherol (vitamin E), carotenoids (beta-carotene, lutein, zeaxanthin, and alpha-carotene) and sulfhydryls.Citation2,Citation3 Flavonoids, coenzyme Q10, alpha-lipoic acid, selenium, pyruvate, and bilirubin are other examples of endogenous non-enzymatic antioxidants. We can also obtain antioxidants exogenously via food intake. Examples of this class of antioxidants, or foods that contain them, are lycopene, curcumin, green tea, Coffea arabica, silymarin, polypodium leucotomos, resveratrol, grape seed extract, pomegranate, pycnogenol, soy isoflavones, propolis, and squalene.Citation4 In skin, the epidermis contains higher concentrations of antioxidants than the dermis. These antioxidants generally are distributed in a gradient fashion with increasing concentrations noted toward the deeper layer of the stratum corneum.Citation5
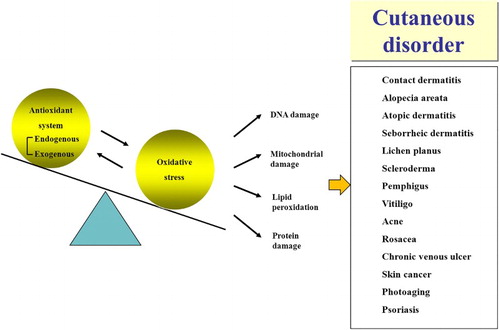
Skin is the largest organ in body that is subjected to oxidative stress, and this stress is known to influence numerous cutaneous diseases (). In this review, we summarize current knowledge regarding oxidative stress and antioxidant strategies in several cutaneous diseases.
Contact dermatitis
Irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) exhibit similar clinical, histological, and molecular features; however, these conditions exhibit different forms of pathogenesis. ICD is a non-immunologic inflammatory reaction in response to toxic materials, whereas ACD is an antigen-specific, memory T-cell-mediated delayed-type hypersensitivity reaction. ROS play a central role in the development of both forms of contact dermatitis.Citation6 The response to irritants or allergens involves the synthesis and release of proinflammatory cytokines as well as the generation of signals to attract leukocytes, upregulate surface costimulatory molecules, activate matrix metalloproteinases (MMPs) and carbonylate proteins. ROS also directly activate or provide costimulatory signals for nuclear factor kappa B (NF-κB), thereby resulting in the regulation of the prostaglandin pathway and the expression of COX-2.Citation7 Thioredoxin is induced by ROS and functions as a chemoattractant for polymorphonuclear leukocytes, monocytes, and T-lymphocytes in inflammatory tissues.Citation8 ROS initially mediate extracellular matrix changes that facilitate ACD. Contact sensitizer-induced, hyaluronidase-mediated hyaluronic acid degradation and sensitization is completely prevented by antioxidants or pre-treatment with the hyaluronidase inhibitor, aristolochic acid.Citation9 Th1 and Th2 response patterns in antigen-presenting cells are modulated by GSH.Citation10
N-Acetylcysteine inhibits IL-1-induced mRNA upregulation and expression of E-selectin and vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells and reduces NF-κB binding to the NF-κB binding site of the VCAM-1 gene.Citation11 N-Acetylcysteine, resveratrol, and tea polyphenols inhibit ROS-regulated expression of MMPs.Citation12 Although N-acetylcysteine, alpha-tocopherol and ascorbate inhibit the expression and functional activity of cutaneous lymphocyte-associated antigen (CLA) in isolated human T-lymphocytes,Citation13 these antioxidants do not inhibit contact dermatitis when administered in topical form.Citation14 In keratinocytes, cell-permeable SOD suppresses TNF-induced MMP-9; thus, SOD is thought to be an immunomodulatory agent in inflammatory skin diseases.Citation15
A topical PPAR-alpha activator induces antioxidant enzymes and reduces inflammation in irritant and ACD models.Citation16
Alopecia areata
Plasma beta-carotene, vitamin E, selenium GPX activity and GSH in plasma and erythrocytes, levels are decreased, whereas thiobarbituric acid reactive substance (TBARS, a marker of lipid peroxidation) in plasma and erythrocytes is increased in alopecia areata (AA) patients.Citation17 GPX and SOD in scalp tissue are increased.Citation18 Given that oxidative stress is associated with cell apoptosis, hair follicle cell apoptosis may be related to oxidative stress in AA. Oxidative stress index (OSI) and total oxidant capacity (TOC) were increased, whereas total antioxidant capacity (TAC) was decreased in AA patients.Citation19
Atopic dermatitis
SOD, catalase, GPX, GSH, and vitamins A, C, and E are decreased in blood of atopic dermatitis patients.Citation20 Urinary 8-hydroxy-2′-deoxyguanosine (a marker of oxidative DNA damage), acrolein-lysine adducts (a marker of lipid peroxidation), and bilirubin oxidative metabolites (a marker of antioxidant activity of bilirubin under oxidative stress) were higher in acute exacerbated atopic dermatitis.Citation21
Seborrheic dermatitis
Serum total antioxidant status (TAS) values were significantly reduced in seborrheic dermatitis patients. However, patients exhibited significantly increased serum total oxidative status (TOS) and OSI values.Citation22 SOD and catalase activities and MDA levels were significantly higher in in scraping samples of patients' scalp.Citation23
Lichen planus
In oral lichen planus, 8-oxo-7,8-dihydro-2′-deoxyguanosine (an indicator of oxidative DNA damage) and 8-nitroguanine (a marker of nitrative DNA damage) in oral epithelium were increased.Citation24 Anthocyanin therapy of oral lichen planus was equal to or better than clobetasol propionate/neomycin/nystatin cream, especially for erosive forms.Citation25
Scleroderma
Oxidative stress is hypothesized to play an important role in disease development. Serum 8-isoprostane (a marker of oxidative stress) was increased in scleroderma patients.Citation26 Similarly, serum TOS and OSI levels indicative of systemic sclerosis were increased and TAC was reduced. Furthermore, TAC may serve as a marker that predicts the risk of lung and gastrointestinal tract involvements.Citation27
Recently, N-acetylcysteine was shown to attenuate skin fibrosis in a bleomycin-induced mouse model of scleroderma; this antioxidant significantly reduced the MDA and protein carbonyl content in the skin of mice.Citation28
Pemphigus vulgaris and pemphigus foliaceus
Serum GPX, catalase and GSH in erythrocyte and plasma, plasma β-carotene, vitamin E, and vitamin A are decreased, whereas MDA in erythrocyte and plasma is increased, in pemphigus vulgaris (PV) patients.Citation29 Serum TOC and lipid hydroperoxide (LOOH) are increased in PV patients.Citation30 Plasma uric acid is reduced, whereas GPX, vitamin C, selenium, and bilirubin levels did not differ in PV patients compared with controls.Citation31
MDA, conjugated dienes, catalase, and SOD activities are increased, whereas protein thiol levels are decreased, in the skin of pemphigus foliaceus (PF) patients.Citation32
Vitiligo
Various conflicting results have been reported with regard to oxidative stress in vitiligo. These differences may be attributed to variations in the innate levels in different tissue samples, disease duration, or disease activity.Citation33
Among ROS, hydrogen peroxide (H2O2) plays a pivotal role in the onset and progression of vitiligo.Citation34 Nuclear transcription factor Nrf2 is thought to mainly regulate heme oxygenase-1 expression, which is involved in protecting human melanocytes against H2O2-induced oxidative stress.Citation35 Nrf2 regulates the expression of various antioxidant enzymes, including catalase, GPX and SOD, via the use of GSH as a substrate. The nuclear factor erythroid 2-related factor 2/antioxidant response element (Nrf2-ARE) pathway is important in protection against oxidative stress-induced cellular injury, and its dysfunction can lead to oxidative stress and melanocyte injury.Citation36 It was recently demonstrated that narrowband ultraviolet (UV) B phototherapy, the well-known treatment of vitiligo, reduces erythrocyte MDA levels and increases GPX levels in patients with vitiligo.Citation37
Acne
SOD and GPX activities are decreased in leukocytes, whereas serum TBARS and MDA are increased, in patients with acne.Citation38 SOD, catalase, GSH, MDA, and adenosine deaminase levels are increased in scrapings of acne tissues.Citation39 Plasma levels of vitamin A and E are decreased in patients with acne.Citation40 Levels of squalene peroxides, which diminish GSH, are increased, whereas vitamin D levels are decreased, in the sebum of acne. Plasma vitamin E, vitamin A, and zinc levels were significantly reduced in acne patients, and a negative correlation between acne severity and vitamin E and zinc levels was noted.Citation41
Among various antioxidants, the vitamin C precursor sodium ascorbyl phosphate, topical or oral zinc, and nicotinamide were proved effective for acne in several studies.Citation42 Other antioxidants that demonstrated efficacy in acne include a multi-nutrient antioxidant capsule consisting of zinc, vitamin C, carotenoids, d-alpha-tocopherol acetate, chromium, selenium and vitamin E, as well as lactoferrin.Citation43
On the other hand, oral isotretinoin treatment for severe acne increases erythrocyte lipid peroxidation, GSH, and GPX and decreases serum paraoxonase-1 activity by increasing oxidative stress. This action was suggested to be a pathomechanism of the side effects of isotretinoin.Citation44
Rosacea
Inflammation is suggested to be associated with ROS produced by inflammatory cells, such as neutrophils in rosacea.Citation45 Serum peroxide and cutaneous ferritin levels are increased, whereas serum total antioxidative potential levels are decreased, in patients with rosacea.Citation46 Effective therapeutic agents for rosacea, including metronidazole, tetracyclines, azelaic acid and azithromycin, exhibit antioxidant activity.Citation47 Oral supplementation of zinc sulfate demonstrated conflicting results regarding the improvement of rosacea.Citation48
Chronic venous ulcer
Oxidative stress potentially results in disturbed wound healing.Citation49 Increases in the allantoin:uric acid percentage ratio (AUR) and 8-isoprostane levels are reported in chronic wounds.Citation50 Selenium, zinc, and iron levels as well as GPX activity are decreased, and ROS-elevated iron deposition and superoxide are increased.Citation51 Increased activity of inducible cyclooxygenase-2 from macrophages and endothelial cells was noted.Citation52 Total iron levels in chronic exudates are also increased,Citation53 along with the proteolytic activity of MMPs and serine proteases.Citation54 SOD, MDA, and NO levels are upregulated in valve tissues, thereby indicating increased oxidative stress.Citation55
UV-associated skin cancer and skin photoaging
ROS can cause DNA damage, which results in mutagenesis and carcinogenesis. Phytochemicals from plant compounds can prevent UV-induced carcinogenesis via their antioxidant function. These compounds also potentially modulate gene expression and signal transduction pathways. Black and green tea, grape seed proanthocyanidins, resveratrol, quercetin, apigenin, silymarin, curcumin, genistein, ascorbic acid and garlic derivatives inhibit or reduce tumorigenesis in murine models or cell lines.
UV induces the generation of ROS and lipid peroxidation products (TBARS) and the depletion of endogenous antioxidants. UV depletes GPX, ascorbate, GSH, SOD, catalase, alpha-tocopherol, and ubiquinol in the skin. Damage to antioxidant systems is more prominent in the epidermis than the dermis.Citation56 It is important to prevent damage to the skin with antioxidants before UV exposure. Antioxidants, including vitamin C, vitamin E, coenzyme Q10, lycopene, carotenoids, tretinoin, GSH, zinc, resveratrol, genistein, cocoa, selenium, and polypodium leucotomos, can exert photoprotective effects. Other antioxidants exhibiting photoprotective properties include melatonin, green tea, silymarin, soy isoflavones, lutein, and zeaxanthin.Citation57
Psoriasis
The imiquimod-induced mouse model of psoriasis exhibits an aberrant antioxidant system. Myeloperoxidase (MPO) and GSH/GSH disulfide (GSSG), one of oxidative stress marker levels are increased, and SOD activity and levels are decreased in mouse skin.Citation58 Cellular signaling pathways, such as mitogen-activated protein kinase/activator protein 1, NF-κB, and Janus kinase-signal transducers, as well as activators of transcription are redox sensitive and involved in the progression of psoriasis.Citation59 Dimethylfumarate upregulates GSH and NAD(P)H:quinone oxidoreductase 1 (NQO1) and activates the Nrf2 transcriptional pathway, thereby resulting in anti-inflammatory effects, such as the downregulation of cytokines and adhesion molecules.Citation60
Imiquimod-induced psoriatic dermatitis exhibits elevated levels of ROS; thus, properly elevated levels of ROS might prevent psoriasis through enhancing indoleamine 2,3-dioxygenase (IDO) expression and Treg function. Successful treatment of psoriasis via hyperbaric oxygen therapy and phototherapy could be attributed to the elevation of ROS levels, which enhances Treg function.Citation61
Conclusion
Although it has been suggested that oxidative stress plays an important role in the development of numerous cutaneous diseases via various redox-sensitive pathways, conflicting results have been reported regarding the oxidant/antioxidant states in these diseases. This discordance can be explained by the following: (1) the innate levels in different tissue samples differ; (2) ROS can affect different complex signaling and biochemical pathways; and (3) oxidative stress can be the result of inflammation, not the cause. Conclusions supporting the efficacy of antioxidative treatments remain still elusive. But there have been many reports about effective antioxidant treatment for cutaneous disease, as well as conventional drugs that have antioxidant activity. Targeting oxidative stress may be an effective strategy for various skin diseases; thus, further studies are required to establish a framework for antioxidative therapeutic plans for each disease.
.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest No potential conflicts of interest relevant to this article are reported.
Ethics approval None.
Acknowledgements
The authors wish to acknowledge the support of the Gachon University, Graduate School of Medicine, and the Gil Hospital Research Foundation.
References
- Chen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermatol 2012;67(5):1013–24. doi: 10.1016/j.jaad.2012.02.009
- Athar M. Oxidative stress and experimental carcinogenesis. Indian J Exp Biol 2002;40(6):656–67.
- Pai VV, Shukla P, Kikkeri NN. Antioxidants in dermatology. Indian Dermatol Online J 2014;5(2):210–4. doi: 10.4103/2229-5178.131127
- Shindo Y, Witt E, Packer L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J Invest Dermatol 1994;102(4):470–5. doi: 10.1111/1523-1747.ep12373027
- Shindo Y, Witt E, Han D, Tzeng B, Aziz T, Nguyen L, et al. Recovery of antioxidants and reduction in lipid hydroperoxides in murine epidermis and dermis after acute ultraviolet radiation exposure. Photodermatol Photoimmunol Photomed 1994;10(5):183–91.
- Kim DH, Byamba D, Wu WH, Kim TG, Lee MG. Different characteristics of reactive oxygen species production by human keratinocyte cell line cells in response to allergens and irritants. Exp Dermatol 2012;21(2):99–103. doi: 10.1111/j.1600-0625.2011.01399.x
- Faux SP, Howden PJ. Possible role of lipid peroxidation in the induction of NF-kappa B and AP-1 in RFL-6 cells by crocidolite asbestos: evidence following protection by vitamin E. Environ Health Perspect 1997;105(Suppl. 5):1127–30.
- Bertini R, Howard OM, Dong HF, Oppenheim JJ, Bizzarri C, Sergi R, et al. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med 1999;189(11):1783–9. doi: 10.1084/jem.189.11.1783
- Agren UM, Tammi RH, Tammi MI. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med 1997;23(7):996–1001. doi: 10.1016/S0891-5849(97)00098-1
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev 2003;8(3):223–46.
- Faruqi RM, Poptic EJ, Faruqi TR, De La Motte C, DiCorleto PE. Distinct mechanisms for N-acetylcysteine inhibition of cytokine-induced E-selectin and VCAM-1 expression. Am J Physiol 1997;273(2 Pt 2):H817–26.
- Polte T, Tyrrell RM. Involvement of lipid peroxidation and organic peroxides in UVA-induced matrix metalloproteinase-1 expression. Free Radic Biol Med 2004;36(12):1566–74. doi: 10.1016/j.freeradbiomed.2004.04.003
- Rutault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med 1999;26(1–2):232–8. doi: 10.1016/S0891-5849(98)00194-4
- Pasche-Koo F, Arechalde A, Arrighi JF, Hauser C. Effect of N-acetylcysteine, an inhibitor of tumor necrosis factor, on irritant contact dermatitis in the human. Curr Probl Dermatol 1995;23:198–206. doi: 10.1159/000424316
- Song HY, Ju SM, Goh AR, Kwon DJ, Choi SY, Park J. Suppression of TNF-alpha-induced MMP-9 expression by a cell-permeable superoxide dismutase in keratinocytes. BMB Rep 2011;44(7):462–7. doi: 10.5483/BMBRep.2011.44.7.462
- Corsini E, Galbiati V, Nikitovic D, Tsatsakis AM. Role of oxidative stress in chemical allergens induced skin cells activation. Food Chem Toxicol 2013;61:74–81. doi: 10.1016/j.fct.2013.02.038
- Naziroglu M, Kokcam I. Antioxidants and lipid peroxidation status in the blood of patients with alopecia. Cell Biochem Funct 2000;18(3):169–73. doi: 10.1002/1099-0844(200009)18:3<169::AID-CBF870>3.0.CO;2-T
- Akar A, Arca E, Erbil H, Akay C, Sayal A, Gür AR. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J Dermatol Sci 2002;29(2):85–90. doi: 10.1016/S0923-1811(02)00015-4
- Bakry OA, Elshazly RM, Shoeib MA, Gooda A. Oxidative stress in alopecia areata: a case-control study. Am J Clin Dermatol 2014;15(1):57–64. doi: 10.1007/s40257-013-0036-6
- Sivaranjani N, Rao SV, Rajeev G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J Clin Diagn Res 2013;7(12):2683–5.
- Tsukahara H, Shibata R, Ohshima Y, Todoroki Y, Sato S, Ohta N, et al. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci 2003;72(22):2509–16. doi: 10.1016/S0024-3205(03)00145-0
- Emre S, Metin A, Demirseren DD, Akoglu G, Oztekin A, Neselioglu S, et al. The association of oxidative stress and disease activity in seborrheic dermatitis. Arch Dermatol Res 2012;304(9):683–7. doi: 10.1007/s00403-012-1254-0
- Ozturk P, Arican O, Belge Kurutas E, Karakas T, Kabakci B. Oxidative stress in patients with scalp seborrheic dermatitis. Acta Dermatovenerol Croat 2013;21(2):80–5.
- Chaiyarit P, Ma N, Hiraku Y, Pinlaor S, Yongvanit P, Jintakanon D, et al. Nitrative and oxidative DNA damage in oral lichen planus in relation to human oral carcinogenesis. Cancer Sci 2005;96(9):553–9. doi: 10.1111/j.1349-7006.2005.00096.x
- Rivarola de Gutierrez E, Di Fabio A, Salomón S, Lanfranchi H. Topical treatment of oral lichen planus with anthocyanins. Med Oral Patol Oral Cir Bucal 2014;19(5):e459–66. doi: 10.4317/medoral.19472
- Avouac J, Borderie D, Ekindjian OG, Kahan A, Allanore Y. High DNA oxidative damage in systemic sclerosis. J Rheumatol 2010;37(12):2540–7. doi: 10.3899/jrheum.100398
- Savas E, Aksoy N, Pehlivan Y, Sayiner ZA, Oztürk ZA, Tabur S, et al. Evaluation of oxidant and antioxidant status and relation with prolidase in systemic sclerosis. Wien Klin Wochenschr 2014;126(11–12):341–6. doi: 10.1007/s00508-014-0534-4
- Zhou CF, Yu JF, Zhang JX, Jiang T, Xu SH, Yu QY, et al. N-acetylcysteine attenuates subcutaneous administration of bleomycin-induced skin fibrosis and oxidative stress in a mouse model of scleroderma. Clin Exp Dermatol 2013;38(4):403–9. doi: 10.1111/ced.12033
- Naziroğlu M, Kökçam I, Simşek H, Karakilçik AZ. Lipid peroxidation and antioxidants in plasma and red blood cells from patients with pemphigus vulgaris. J Basic Clin Physiol Pharmacol 2003;14(1):31–42. doi: 10.1515/JBCPP.2003.14.1.31
- Yesilova Y, Ucmak D, Selek S, Dertlioğlu SB, Sula B, Bozkus F, et al. Oxidative stress index may play a key role in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol 2013;27(4):465–7. doi: 10.1111/j.1468-3083.2012.04463.x
- Yousefi M, Rahimi H, Barikbin B, Toossi P, Lotfi S, Hedayati M, et al. Uric acid: a new antioxidant in patients with pemphigus vulgaris. Indian J Dermatol 2011;56(3):278–81. doi: 10.4103/0019-5154.82480
- Abida O, Gargouri B, Ben Mansour R, Mseddi-Djemal M, Masmoudi A, Ben Ayed M, et al. Biomarkers of oxidative stress in epidermis of Tunisian pemphigus foliaceus patients. J Eur Acad Dermatol Venereol 2013;27(3):e271–5. doi: 10.1111/j.1468-3083.2012.04626.x
- Jian Z, Li K, Song P, Zhu G, Zhu L, Cui T, et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in Vitiligo. J Invest Dermatol 2014;134(8):2221–30. doi: 10.1038/jid.2014.152
- Schallreuter KU, Gibbons NC, Zothner C, Abou Elloof MM, Wood JM. Hydrogen peroxide-mediated oxidative stress disrupts calcium binding on calmodulin: more evidence for oxidative stress in vitiligo. Biochem Biophys Res Commun 2007;360(1):70–5. doi: 10.1016/j.bbrc.2007.05.218
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003;3(10):768–80. doi: 10.1038/nrc1189
- Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 2010;298(3):F662–71. doi: 10.1152/ajprenal.00421.2009
- Karsli N, Akcali C, Ozgoztasi O, Kirtak N, Inaloz S. Role of oxidative stress in the pathogenesis of vitiligo with special emphasis on the antioxidant action of narrowband ultraviolet B phototherapy. J Int Med Res 2014;42(3):799–805. doi: 10.1177/0300060513516294
- Basak PY, Gultekin F, Kilinc I. The role of the antioxidative defense system in papulopustular acne. J Dermatol 2001;28(3):123–7. doi: 10.1111/j.1346-8138.2001.tb00105.x
- Perihan O, Ergul KB, Neslihan D, Filiz A. The activity of adenosine deaminase and oxidative stress biomarkers in scraping samples of acne lesions. J Cosmet Dermatol 2012;11(4):323–8. doi: 10.1111/jocd.12011
- El-Akawi Z, Abdel-Latif N, Abdul-Razzak K. Does the plasma level of vitamins A and E affect acne condition? Clin Exp Dermatol 2006;31(3):430–4. doi: 10.1111/j.1365-2230.2006.02106.x
- Ozuguz P, Dogruk Kacar S, Ekiz O, Takci Z, Balta I, Kalkan G. Evaluation of serum vitamins A and E and zinc levels according to the severity of acne vulgaris. Cutan Ocul Toxicol 2014;33(2):99–102. doi: 10.3109/15569527.2013.808656
- Bowe WP, Patel N, Logan AC. Acne vulgaris: the role of oxidative stress and the potential therapeutic value of local and systemic antioxidants. J Drugs Dermatol 2012;11(6):742–6.
- Gori A, Fabroni C, Prignano F, Lotti T. Unusual presentation of tuberculosis in an infliximab-treated patient--which is the correct TB screening before starting a biologic?. Dermatol Ther 2010;23(Suppl. 1):S1–3. doi: 10.1111/j.1529-8019.2009.01278.x
- Erturan İ, Naziroğlu M, Akkaya VB. Isotretinoin treatment induces oxidative toxicity in blood of patients with acne vulgaris: a clinical pilot study. Cell Biochem Funct 2012;30(7):552–7. doi: 10.1002/cbf.2830
- Jones D. Reactive oxygen species and rosacea. Cutis 2004;74(3 Suppl):17–20, 32–4.
- Tisma VS, Basta-Juzbasic A, Jaganjac M, Brcic L, Dobric I, Lipozencic J, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol 2009;60(2):270–6. doi: 10.1016/j.jaad.2008.10.014
- Bakar O, Demirçay Z, Yuksel M, Haklar G, Sanisoglu Y. The effect of azithromycin on reactive oxygen species in rosacea. Clin Exp Dermatol 2007;32(2):197–200. doi: 10.1111/j.1365-2230.2006.02322.x
- Bamford JT, Gessert CE, Haller IV, Kruger K, Johnson BP. Randomized, double-blind trial of 220 mg zinc sulfate twice daily in the treatment of rosacea. Int J Dermatol 2012;51(4):459–62. doi: 10.1111/j.1365-4632.2011.05353.x
- Sharquie KE, Najim RA, Al-Salman HN. Oral zinc sulfate in the treatment of rosacea: a double-blind, placebo-controlled study. Int J Dermatol 2006;45(7):857–61. doi: 10.1111/j.1365-4632.2006.02944.x
- James TJ, Hughes MA, Cherry GW, Taylor RP. Evidence of oxidative stress in chronic venous ulcers. Wound Repair Regen 2003;11(3):172–6. doi: 10.1046/j.1524-475X.2003.11304.x
- Agren MS, Strömberg HE, Rindby A, Hallmans G. Selenium, zinc, iron and copper levels in serum of patients with arterial and venous leg ulcers. Acta Derm Venereol 1986;66(3):237–40.
- Abd-El-Aleem SA, Ferguson MW, Appleton I, Bhowmick A, McCollum CN, Ireland GW. Expression of cyclooxygenase isoforms in normal human skin and chronic venous ulcers. J Pathol 2001;195(5):616–23. doi: 10.1002/path.992
- Wenk J, Foitzik A, Achterberg V, Sabiwalsky A, Dissemond J, Meewes C, et al. Selective pick-up of increased iron by deferoxamine-coupled cellulose abrogates the iron-driven induction of matrix-degrading metalloproteinase 1 and lipid peroxidation in human dermal fibroblasts in vitro: a new dressing concept. J Invest Dermatol 2001;116(6):833–9. doi: 10.1046/j.1523-1747.2001.01345.x
- Grinnell F, Ho CH, Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol 1992;98(4):410–6. doi: 10.1111/1523-1747.ep12499839
- Karatepe O, Unal O, Ugurlucan M, Kemik A, Karahan S, Aksoy M, et al. The impact of valvular oxidative stress on the development of venous stasis ulcer valvular oxidative stress and venous ulcers. Angiology 2010;61(3):283–8. doi: 10.1177/0003319709343177
- Shindo Y, Witt E, Packer L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J Invest Dermatol 1994;102(4):470–5. doi: 10.1111/1523-1747.ep12373027
- Goswami S, Haldar C. Melatonin improves UVB induced oxidative damages and inflammatory conditions of cutaneous tissue of a diurnal Indian palm squirrel Funambulus pennant. Br J Dermatol 2014;171(5):1147–55. doi: 10.1111/bjd.13117
- Baek JO, Byamba D, Wu WH, Kim TG, Lee MG. Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch Dermatol Res 2012;304(9):699–706. doi: 10.1007/s00403-012-1272-y
- Zhou Q, Mrowietz U, Rostami-Yazdi M. Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med 2009;47(7):891–905. doi: 10.1016/j.freeradbiomed.2009.06.033
- Begleiter A, Sivananthan K, Curphey TJ, Bird RP. Induction of NAD(P)H quinone: oxidoreductase1 inhibits carcinogen-induced aberrant crypt foci in colons of Sprague-Dawley rats. Cancer Epidemiol Biomarkers Prev 2003;12(6):566–72.
- Kim HR, Lee A, Choi EJ, Hong MP, Kie JH, Lim W, et al. Reactive oxygen species prevent imiquimod-induced psoriatic dermatitis through enhancing regulatory T cell function. PLos One 2014;9(3):e91146. doi: 10.1371/journal.pone.0091146