Abstract
Background: The aim of this study was to investigate oxidative stress and thiol/disulfide status with a novel automated homeostasis assay in advanced non-small cell lung cancer (NSCLC).
Methods: Thirty-five patients with advanced NSCLC, who had been newly diagnosed and previously untreated, and 35 healthy subjects were chosen for the study. We measured plasma total thiol (–SH+–S–S–), native thiol (thiol) (–SH), and disulfide (–S–S–) levels in the patients with NSCLC and the healthy subjects. The thiol/disulfide (–SH/–S–S–) ratio was also calculated.
Results: Statistically significant differences between the patient group and the control group were detected for the thiol/disulfide parameters. The mean native thiol, total thiol, and disulfide levels were significantly lower in the group with advanced stage NSCLC. The cut-off value was 313 and 13.8 for native thiol and disulfide, respectively. Median overall survival (OS) was significantly shorter in patients with low native thiol and disulfide levels according to the cut-off value (respectively, P = 0.001; P = 0.006). Native thiol, total thiol, and disulfide levels were correlated with Karnofsky performance status (KPS), OS, and age. Additionally, hierarchical regression analyses showed gender, KPS, lung metastases, and plasma native thiol levels were the determinants of OS in the final model.
Conclusion: These results suggest that in advanced stage NSCLC, the native thiol, total thiol, and disulfide levels decrease, while the native thiol/disulfide ratio does not change. Low levels of thiol/disulfide parameters are related to tumor aggressiveness and may predict a poor outcome for patients with NSCLC.
Introduction
Lung cancer is one of the most common malignant tumors and the leading cause of cancer-related death worldwide. Non-small cell lung cancer (NSCLC) accounts for 80–85% of all lung cancer cases and its 5-year survival rate is very poor (about 15%).Citation1
Reactive oxygen species (ROS) are physiologically produced by aerobic cells, and production increases during cell damage.Citation2 Physiological levels of ROS mediate critical intracellular survival signaling pathways. In addition, ROS, present in inflamed tissue, can cause the inflammatory process; thus, ROS have great significance in tumorigenesis.Citation3 Oxidative stress induces a cellular redox imbalance which has been found to be present in various cancer cells compared with normal cells; the redox imbalance thus may be related to oncogenic stimulation.Citation4
Oxidative stress occurs when ROS levels exceed the antioxidant capacity of a cell.Citation2 Lung cancer cells can grow in an excessively oxidative environment,Citation5–Citation7 which is believed to contribute to tumor progress and metastasis.Citation8 High levels of oxidative markers are related to tumor aggressiveness and can predict an unfavorable outcome for patients with early-stage lung adenocarcinoma.Citation9 Moreover, clinical data have shown that patients with lung cancer have increased oxidative stress markers in peripheral blood, erythrocytes, epithelium lining fluid, breath condensate, and tumor biopsies, and insufficient ingestion of antioxidants constitutes a risk factor for developing lung cancer.Citation5–Citation7,Citation10 Although lung cells have reduction/oxidation (redox) features, the effect of oxidative stress in lung cancer biology is poorly understood.
The effects of ROS are balanced by the action of enzymatic and non-enzymatic antioxidant systems. The enzymatic antioxidants (e.g. superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX)), lung tissue expresses several thiol-containing proteins and small molecules, including thioredoxins, metallothioneins, glutathione (GSH), and peroxidases such as thioredoxin reductases and peroxiredoxins (also called thioredoxin peroxidases), which all contain the amino acid cysteine in their active centers.Citation9
The non-enzymatic antioxidants, compounds that have sulfhydryl groups (–SH), called thiols (R–SH), are particularly important.Citation11 Total thiols consist of intracellular and extracellular thiols either in the free form or reduced GSH, or thiols bound to proteins. The plasma thiol pool is chiefly composed of albumin, protein, and low-molecular-weight thiols.Citation12 Thiols contribute the major portion of the total antioxidants present in the body and play an important role in defense against ROS.Citation13 Measuring thiols in serum provides an indirect reflection of the antioxidative defense.Citation14 Thiols can form disulfide bonds with oxidation reactions. The disulfide bonds can again be reduced to thiols, and thus, dynamic thiol–disulfide homeostasis. Dynamic thiol–disulfide homeostasis status has critical roles in antioxidant protection, detoxification, signal transduction, apoptosis, regulation of enzymatic activity and transcription factors, and cellular signaling mechanisms.Citation15 An abnormal thiol–disulfide homeostasis state is involved in the pathogenesis of various diseases, including diabetes, cardiovascular disease, cancer, rheumatoid arthritis, chronic kidney disease, acquired immunodeficiency syndrome, Parkinson's disease, Alzheimer's disease, Friedreich's ataxia, multiple sclerosis and amyotrophic lateral sclerosis, and liver disorder.Citation16
Thus, we aimed to investigate the associations between clinicopathological parameters and survival of patients with NSCLC with oxidative stress with a novel specific automated thiol/disulfide homeostasis assay.
Materials and methods
Patients and healthy subjects
The 35 patients included in this study were newly diagnosed with advanced stage (IIIB and IV) with histologically or cytologically confirmed NSCLC. Disease staging was performed according to the seventh edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. At the beginning of the study, the patients had not been treated with anticancer medication and had not undergone radiotherapy. Some patients had received platinum-based chemotherapy after diagnosis. For prognosis analyses, the overall survival (OS) was calculated from the date of diagnosis to the date of last follow-up or death from any cause. In addition, the Karnofsky performance status (KPS) was recorded for the patients. The control group was composed of 35 healthy individuals. In the patient and control groups, patients with active infection, chronic inflammatory or autoimmune disease, and drug intake and steroid treatment were excluded from the study.
Blood samples
Peripheral blood samples (5 ml) were obtained from healthy subjects and at the time of diagnosis with patients. Samples were collected in tubes containing EDTA. Plasma samples were separated from cells by centrifugation at 1500 × g for 10 minutes and were stored at −80°C.
Assay
Principle of the new assay
Dynamic disulfide bonds (–S–S–) in the sample are reduced to functional thiol groups (–SH) by NaBH4. The unused NaBH4 remnants are completely removed by formaldehyde. Thus, this prevents the extra reduction of the DTNB and further reduction of the formed disulfide bond, which are produced after the DTNB reaction. The total thiol content of the sample is measured using modified Ellman reagent. Native thiol content is subtracted from the total thiol content and half of the obtained difference gives the disulfide bond amount. The disulfide parameter is a value which can be calculated automatically as half of the difference of the two measured values. The assays can also be performed manually by using spectrophotometers or multiwell readers. All volumes of the samples and reagents must be increased at the same ratio. Use of a second (side) wavelength is optional.
In the first step of the assay, only dynamic and reducible disulfide bonds were completely reduced to free functional thiol groups. Static and structural disulfide bonds were not. In the second step, the remaining, unused reductant NaBH4, was completely consumed, and in the last step, all thiol groups – consisting of native thiol groups plus reduced thiol groups – were reacted with DTNB. Half of the difference between assay pairs gave disulfide amounts, which were calculated automatically. In addition to the measurement of plasma native thiol and disulfide amounts, –S–S–/–SH, –S–S–/(–SH+–S–S–), and –SH/(–SH+–S–S–) ratios were calculated automatically and synchronously.Citation16
Optimizations of NaBH4 and formaldehyde concentrations
Pure sodium borohydrate solution reduced DTNB to TNB and led to false positive results in the assay. It also reduced the formed disulfide bonds, which are produced after DTNB reduction to TNB, to free sulfhydryl groups; this vicious circle proceeded until NaBH4 was consumed. After the reduction of dynamic disulfide bonds to free sulfhydryl groups, the unused NaBH4 remnants were completely removed using formaldehyde. After the removal of NaBH4 by formaldehyde, no false positive result interference was seen; thus, the extra reduction of DTNB and further reduction of the formed disulfide bonds were completely prevented. Optimal NaBH4 concentration was found to be 10 mM. The lower concentrations showed insufficient reduction capacity for disulfide bonds, while the higher concentrations showed false positive results. Optimal concentrations of the chemicals used were determined by changing the concentration of one chemical while the concentrations of the other chemicals were kept constant. The optimal formaldehyde concentration was found to be a millimolar concentration of 6.715.
Interference
It was found that hemoglobin, EDTA, citrate, and oxalate did not interfere with the assay developed, but bilirubin did negatively interfere with the assay. Lipemic and uremic plasma samples did not interfere with the assay. Plasma and serum samples can be used as samples.
Statistical analysis
Analyses were performed using SPSS software (version 22.0, SPSS, Chicago, IL). P values <0.05 were considered statistically significant. The Kolmogorov–Smirnov test was used to evaluate the normality of the distributions of variables. Parametric continuous variables were presented as mean ± standard deviations. Differences in the continuous variables between groups were assessed by t-test for variables with normal distribution or the Mann–Whitney U-test for variables without normal distribution. Categorical variables were summarized as frequencies and compared with the chi-square test. The Spearman or Pearson correlation coefficient was computed to examine the association between two continuous variables. The levels of plasma thiol and disulfide values in predicting presence of NSCLC disease were analyzed using receiver operating characteristics (ROC) curve analysis. In cases where the ROC curve analysis yielded a significant cut-off value, the sensitivity, specificity, positive, and negative predictive values were calculated. When evaluating the area under the curve, a 5% type-1 error level was used to accept a statistically significant predictive value of the test variables. OS probability was calculated using the product limit method of Kaplan–Meier. Differences in survival between groups were determined using the log-rank test. A multiple linear regression model was used to identify independent predictors of OS.
Results
The demographics and the thiol/disulfide homeostasis parameters (native thiol, disulfide, total thiol, and native thiol/disulfide) of the patients and healthy controls are summarized in Table . There were no significant differences between the age and gender of the patients and the healthy controls (P = 0.882 and P = 0.784, respectively). The native thiol, disulfide, and total thiol levels were statistically significantly decreased (P < 0.05); however, the native thiol/disulfide ratio was not statistically significant in the patients with NSCLC compared with the control subjects. The clinicopathological features of the patients are presented in Table : the histological type, KPS, tumor spread (cT), lymph node metastasis (cN), treatment status (chemotherapy, radiotherapy), and location and number of distant metastases.
Table 1 Characteristics of subjects
Table 2 Patients characteristics
Total and native thiol levels correlated negatively with KPS (P < 0.05). The native thiol levels correlated positively with OS, disulfide, total thiol, and native thiol/disulfide (P < 0.05 for OS and disulfide; P < 0.01 for total thiol and native thiol/disulfide). In addition, the disulfide levels were negatively correlated with native thiol/disulfide (P < 0.01). However, the disulfide levels were positive correlated with OS, native thiol, and total thiol (P < 0.05 for all). These data are summarized in Table .
Table 3 Means, standard deviations, and correlations among variables
Hierarchical regression analyses were conducted to test the relative explanatory values of three sets of variables on OS: (1) the demographics (gender, age); (2) clinicopathological features (KPS, cN, cT, metastasis status, and site number); (3) plasma marker levels (thiol, total thiol, and disulfide). The demographic factors were first controlled in Block 1. Clinicopathological features were entered in Block 2, and plasma marker levels were entered in Block 3. The results showed that gender, KPS, lung metastases, and plasma native thiol levels were the determinants of OS in the final model (Table ).
Table 4 Results of the regression analysis with OS as criterion variable
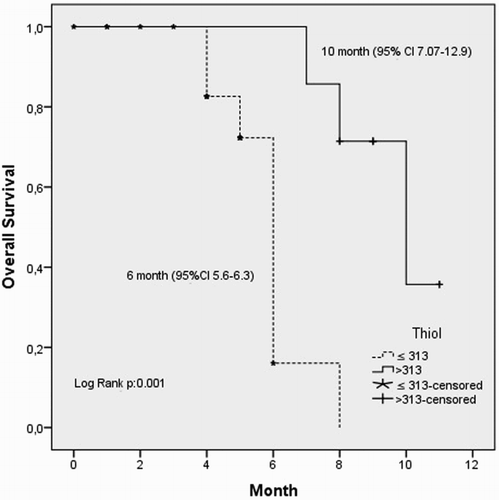
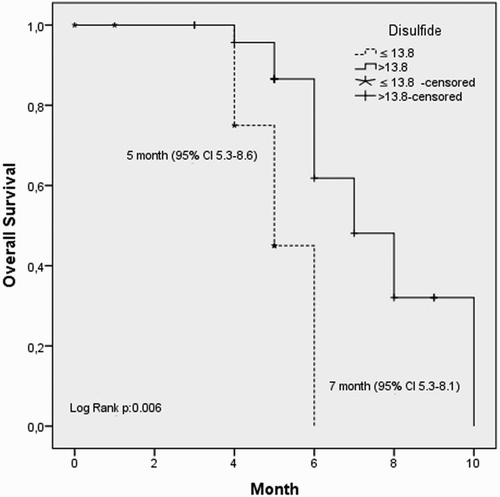
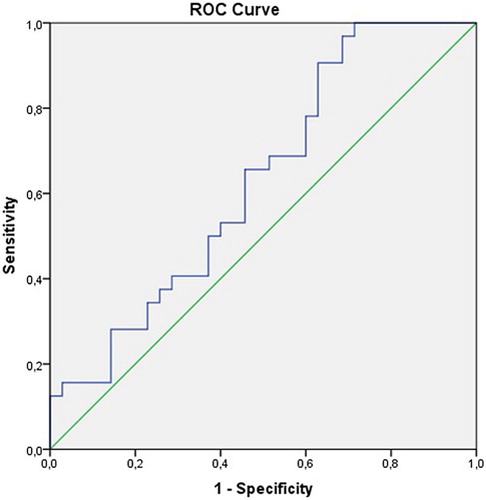
We defined the ROC curves of the plasma levels of thiol and disulfide in relation to disease presence of NSCLC. The cut-off point for the plasma thiol and disulfide levels was 313 (sensitivity = 80%, specificity = 60%) and 13.8 (sensitivity = 30%, specificity = 99%), respectively (area under curve = 0.69 [95% confidence interval, CI = 0.56–0.82] and 0.62 [95% CI = 0.49–0.76], respectively); see Figs. and . If the patients' thiol and disulfide levels were above or equal to the estimated cut-off points, the levels were high, and if the level was below the cut-off point, the level was low. The percentage of patients with low thiol levels was 80% and with low disulfide levels was 28.6%.
Figure 1 The ROC curve showing the sensitivity and specificity of the plasma thiol levels due to disease development of NSCLC.
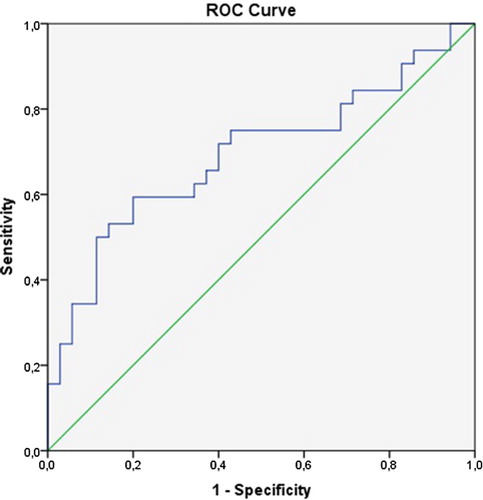
Figure 2 The ROC curve showing the sensitivity and specificity of the plasma disulfide levels due to disease development of NSCLC.
When we recategorized the patients regarding high or low plasma markers and compared them statistically, we found a significant correlation between these parameters and OS. The median OS (10 vs. 6 months, P = 0.001, log-rank test) was significantly shorter in patients with low thiol levels (Fig. ). Similar results were obtained for low disulfide levels (median OS; 7 vs. 5 months, P = 0.006, log-rank test, Fig. ).
Discussion
To our knowledge, this was the first study that examined the relationships between oxidative status, clinicopathological features, and OS in patients with NSCLC with a new automated assay, thiol/disulfide homeostasis. We showed that the native thiol, disulfide, and total thiol levels in patients with NSCLC were decreased statistically significantly compared with those of the control subjects. Our findings suggest that low plasma native thiol and disulfide levels were correlated with inferior OS. Additionally, the plasma native thiol, total thiol, and disulfide levels correlated with KPS, age, and OS in patients with NSCLC. Furthermore, low KPS, male gender, lung metastases, and low native thiol levels are potential risk factors for poor OS of patients with NSCLC.
The redox status related to the production of intracellular ROS in cancer cells has also been denoted to control the aggression of cancer cells. Chemically, oxidative stress is associated with the increased production of oxidizing species or a significant decrease in the effectiveness of antioxidant defenses and repair systems. Induced oxidative stress in cancer cells is a result of the lower levels of antioxidant enzymes such as SOD, GPX, GSH reductase, and CAT.Citation17 In addition, in many malignancies, footprints of oxidative damage, the markers of the imbalance between oxidants and antioxidants, have been detected and associated with several cancer-related processes, such as resistance to chemotherapy.Citation15,Citation18–Citation21
The action of non-enzymatic antioxidants, such as total thiols and non-protein thiol groups, are extremely important because they contribute to protecting normal cell structures and functions, since –SH groups are included in maintaining redox homeostasis, extinguishing free radicals, and participating in detoxification reactions.Citation22 Zanini et al.Citation23, in patients with lung cancer, investigated the activity of the enzymes included in oxidative stress status such as SOD and CAT, and verified the total thiols, non-protein thiols, and thiobarbituric acid reactive substances (TBARS: markers of lipid peroxidation) levels. The antioxidant enzymes SOD and CAT, as well as the total thiol and non-protein thiol levels, were higher and TBARS levels were lower in the patients with lung cancer compared with the control group. However, Klarod et al.Citation24 found the non-enzymatic antioxidant levels, including retinol, alpha-tocopherol, beta-carotene, lycopene, beta-cryptoxanthin, selenium, and zinc, were lower in patients with lung cancer than in the control group, especially in patients with advanced stage disease. In addition, Gupta et al.Citation25 showed that oxidative/antioxidative parameters for controls and patients with NSCLC such as GSH levels and SOD activity were significantly lower in the patients with NSCLC compared with the control subjects. These results suggested that a decrease in antioxidant enzyme activity may be a response to increased ROS production, and in time antioxidant enzyme activity may be insufficient for detoxification of high levels of ROS.
There is no agreement about the level of antioxidants in lung cancer. Studies have shown increased antioxidant activity in tumor cells or the lung epithelial lining fluid of individuals with lung cancer while others have reported reduced antioxidant activity in lung tumors.
In the current study, the native thiol, disulfide, and total thiol levels were decreased statistically significantly in patients with NSCLC compared with the control subjects while the native thiol/disulfide ratio does not change (Table ). Under oxidative stress disulfide level is expected to increase as thiol level decreases. But results of our study did not support this. There are many factors affecting the thiol–disulfide balance except oxidative stress and these factors may have affected the results. Thiol and disulfide are not stable due to the plasma kinetic barriers. The steady state concenrations and also the ratios of oxidized versus reduced thiols are results of several concurrent processes. Processes that affect the concentrations of thiols and oxidized are; the rates of the thiol-disulfide exchange reactions, the rates of thiol oxidation by ROS and possible repair processes, the rates of enzymatic extracellular degradation of GSH, the rates of transport between the plasma compartment and especially erythrocytes and endothelial cells, rates of liver release of thiol-containing molecules such as human serum albumin, glutathione, according to a minor extent rates of renal excretion and intracellular metabolism. However, certain quantitative information about the process is not clear.Citation12
Our study cut-off value was determined using ROC analysis for native thiol and disulfide levels in terms of showing the disease (Figs. and ). Accordingly, the sensitivity of the thiol levels and the specificity of the disulfide levels were high (80%, 99%, respectively). In previous studies, cut-off values of thiol/disulfide homeostasis were not determined for the diagnosis of lung cancer. The low antioxidant levels (i.e. TBARS levels) in patients with lung cancer could be related to the increasing tumor size and progression of cancer.Citation26,Citation27 Gupta et al.Citation25 showed that after standard platinum-based chemotherapy in patients with lung cancer, GSH levels increased significantly in responders compared with non-responders. These effects may also contribute to increased survival among responders. According to this study, the low GSH levels were associated with a poor outcome. In another study, a decrease in antioxidant enzymatic activity in the cancerous and borderline regions promoted the growth of cancer tissue and its infiltration into the surrounding tissues, which is important in invasion and metastasis.Citation28
It is shown that increased ROS products or decreased antioxidant levels cause DNA damage and this is related with short survival times of patients with cancer.Citation29 Similarly, we found low native thiol and disulfide levels were associated with poor OS (Figs. and ). We also found a positive correlation between thiol/disulfide homeostasis parameters (Table ). The linear regression analysis showed that plasma native thiol levels are independent from OS (Table ). In this analysis, we observed that other factors that identify OS are the male gender, KPS, and lung metastases.
A relation between oxidative stress and cachexia and poor performance status has been shown in many cancers including lung cancer.Citation24,Citation30–Citation32 Oxidative stress, the main feature of cancer cachexia, triggers the overproduction of ROS or decreases antioxidant playing a major role in the pathogenesis of systemic symptoms of cachexia.Citation33 In our study, there was a correlation between the KPS score and low thiol/disulfide homeostasis parameters.
Conclusion
In summary, the thiol/disulfide homeostasis levels measured with the new method were low and were related to KPS and poor OS. To our knowledge, this is the first study of oxidative status with a novel automated assay thiol/disulfide status as a possible prognostic marker. The limitation is that the small number of cases in each group barely produced statistically significant results. Further studies are needed to investigate the interaction between oxidants and antioxidants in lung cancer as well as the impact on the clinical outcome or the treatment interference.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflicts of interest The authors have no conflicts of interest to declare.
Ethics approval The study was conducted according to Good Clinical Practice and the Declaration of Helsinki. Protocol approval was obtained from an independent Ethics Committee at Suleyman Demirel University (protocol number: 72867572-050-157). All patients provided written informed consent.
References
- Kocaturk C, Gunluoglu MZ, Cansever L, Dincer IS, Bedirhan MA. Prognosis in patients with non-small cell lung cancer and satellite tumors. Thorac Cardiovasc Surg 2011;59(6):360–3. doi: 10.1055/s-0030-1270799
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001
- Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev 2008;11(1):1–15. doi: 10.1080/10937400701436460
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001
- Brennan P, Fortes C, Butler J, Agudo A, Benhamou S, Darby S, et al. A multicenter case–control study of diet and lung cancer among non-smokers. Cancer Causes Control 2000;11(1):49–58. doi: 10.1023/A:1008909519435
- Esme H, Cemek M, Sezer M, Saglam H, Demir A, Melek H, et al. High levels of oxidative stress in patients with advanced lung cancer. Respirology 2008;13(1):112–6. doi: 10.1111/j.1440-1843.2007.01212.x
- Chan HP, Tran V, Lewis C, Thomas PS. Elevated levels of oxidative stress markers in exhaled breath condensate. J Thorac Oncol 2009;4(2):172–8. doi: 10.1097/JTO.0b013e3181949eb9
- Sotgia F, Martinez-Outschoorn UE, Lisanti MP. Mitochondrial oxidative stress drives tumor progression and metastasis: should we use antioxidants as a key component of cancer treatment and prevention? BMC Med 2011;9:62. doi: 10.1186/1741-7015-9-62
- da Motta LL, Müller CB, de Bastiani MA, Behr GA, França FS, da Rocha RF, et al. Imbalance in redox status is associated with tumor aggressiveness and poor outcome in lung adenocarcinoma patients. J Cancer Res Clin Oncol 2014;140(3):461–70. doi: 10.1007/s00432-014-1586-6
- Blair SL, Heerdt P, Sachar S, Abolhoda A, Hochwald S, Cheng H, et al. Glutathione metabolism in patients with non-small cell lung cancers. Cancer Res 1997;57(1):152–55.
- Karoui H, Hogg N, Fréjaville C, Tordo P, Kalyanaraman B. Characterization of sulfur-centered radical intermediates formed during the oxidation off thiols and sulfite by peroxynitrite – ESR-SPIN trapping and oxygen uptake studies. J Biol Chem 1996;271:6000–9. doi: 10.1074/jbc.271.11.6000
- Turell L, Radi R, Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med 2013;65:244–53. doi: 10.1016/j.freeradbiomed.2013.05.050
- Chianeh YR, Prabhu K. Protein thiols as an indicator of oxidative stress. Arch Med Rev J 2014;23(3):443–56.
- Taysi S, Polat F, Gul M, Sari RA, Bakan E. Lipid peroxidation, some extracellular antioxidants, and antioxidant enzymes in serum of patients with rheumatoid arthritis. Rheumatol Int 2002;21(5):200–4. doi: 10.1007/s00296-001-0163-x
- Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 2010;48(6):749–62. doi: 10.1016/j.freeradbiomed.2009.12.022
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulfide homeostasis. Clin Biochem 2014;47(18):326–32. doi: 10.1016/j.clinbiochem.2014.09.026
- Güner G, Işlekel H, Oto O, Hazan E, Açikel U. Evaluation of some antioxidant enzymes in lung carcinoma tissue. Cancer Lett 1996;103(2):233–9. doi: 10.1016/0304-3835(96)04226-7
- Yoo DG, Song YJ, Cho EJ, Lee SK, Park JB, Yu JH, et al. Alteration of APE1/ref-1 expression in non-small cell lung cancer: the implicationsof impaired extracellular superoxide dismutase and catalase antioxidant systems. Lung Cancer 2008;60(2):277–84. doi: 10.1016/j.lungcan.2007.10.015
- Wang D, Xiang DB, Yang XQ, Chen LS, Li MX, Zhong ZY, et al. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells. Lung Cancer 2009;66(3):298–304. doi: 10.1016/j.lungcan.2009.02.019
- Marikovsky M, Nevo N, Vadai E, Harris-Cerruti C. Cu/Zn superoxide dismutase plays a role in angiogenesis. Int J Cancer 2002;97(1):34–41. doi: 10.1002/ijc.1565
- Pani G, Galeotti T, Chiarugi P. Metastasis: cancer cell's escape from oxidative stress. Cancer Metastasis Rev 2010;29(2):351–78. doi: 10.1007/s10555-010-9225-4
- De Bona KS, Bellé LP, Bittencourt PE, Bonfanti G, Cargnelluti LO, Pimentel VC, et al. Erythrocytic enzymes and antioxidant status in people with type 2 diabetes: beneficial effect of Syzygium cumini leaf extract in vitro. Diabetes Res Clin Pract 2011;94(1):84–90. doi: 10.1016/j.diabres.2011.06.008
- Zanini D, Schmatz R, Pelinson LP, Pimentel VC, Costa P, Cardoso AM, et al. Ectoenzymes and cholinesterase activity and biomarkers of oxidative stress in patients with lung cancer. Mol Cell Biochem 2013;374(1–2):137–48. doi: 10.1007/s11010-012-1513-6
- Klarod K, Hongsprabhas P, Khampitak T, Wirasorn K, Kiertiburanakul S, Tangrassameeprasert R, et al. Serum antioxidant levels and nutritional status in early and advanced stage lung cancer patients. Nutrition 2011;27(11–12):1156–60. doi: 10.1016/j.nut.2010.12.019
- Gupta A, Srivastava S, Prasad R, Natu SM, Mittal B, Negi MP, et al. Oxidative stress in non-small cell lung cancer patients after chemotherapy: association with treatment response. Respirology 2010;15(2):349–56. doi: 10.1111/j.1440-1843.2009.01703.x
- Seven A, Erbil Y, Seven R, Inci F, Gülyasar T, Barutcu B, et al. Breast cancer and benign breast disease patients evaluated in relation to oxidative stress. Cancer Biochem Biophys 1998;16(4):333–45.
- Gerber M, Astre C, Segala C, Saintot M, Scali J, Simony-Lafontaine J, et al. Oxidant–antioxidant status alterations in cancer patients: relationship to tumor progression. J Nutr 1996;126(4):1201–7.
- Manabe T, Asano N, Yoshimura T, Suwa H, Imamura T, Ohshio G. Effect of synthetic protease inhibitor on histologic changes and free radical activity in hamsters with pancreas cancer. Scand J Gastroenterol 1993;28(8):719–24. doi: 10.3109/00365529309098280
- Thanan R, Pairojkul C, Pinlaor S, Khuntikeo N, Wongkham C, Sripa B, et al. Inflammation-related DNA damage and expression of CD133 and Oct3/4 in cholangiocarcinoma patients with poor prognosis. Free Radic Biol Med 2013;65:1464–72. doi: 10.1016/j.freeradbiomed.2013.07.034
- Mantovani G, Macciò A, Madeddu C, Mura L, Gramignano G, Lusso MR, et al. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med 2003;81(10):664–73. doi: 10.1007/s00109-003-0476-1
- Macciò A, Madeddu C, Gramignano G, Mulas C, Floris C, Sanna E, et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol 2012;124(3):417–25. doi: 10.1016/j.ygyno.2011.12.435
- Mantovani G, Macciò A, Madeddu C, Mura L, Gramignano G, Lusso MR, et al. Quantitative evaluation of oxidative stress, chronic inflammatory indices and leptin in cancer patients: correlation with stage and performance status. Int J Cancer 2002;98(1):84–91. doi: 10.1002/ijc.10143
- Mantovani G, Madeddu C. Cyclooxygenase-2 inhibitors and antioxidants in the treatment of cachexia. Curr Opin Support Palliat Care 2008;2(4):275–81. doi: 10.1097/SPC.0b013e32830f47e4