Abstract
Objectives: Sepsis is associated with oxidative stress. Due to oxidative stress, three tyrosine isoforms, para-, meta-, and ortho-tyrosine (p-, m-, and o-Tyr), can be formed non-enzymatically in smaller amounts. p-Tyr is mainly formed physiologically in the kidneys through the activity of the phenylalanine hydroxylase enzyme. The three tyrosine isoforms may undergo different renal handling.
Methods: Twenty septic patients were involved in the study and 25 healthy individuals served as controls. Blood and urine levels of p-, m-, and o-Tyr were measured on admission and four consecutive days.
Results: Serum m-Tyr levels were higher in septic patients than in controls on days 2 (P = 0.031) and 3 (P = 0.035). Serum p-Tyr levels were lower in the cases than in controls on days 1 (P = 0.005) and 2 (P = 0.040), and subsequently normalized due to a day-by-day elevation (P = 0.002). The tendency of urinary m-Tyr concentration was decreasing (P = 0.041), while that of urinary p-Tyr concentration was increasing (P = 0.001). Fractional excretion of m-Tyr (FEm-Tyr) showed a decreasing tendency (P = 0.009), and was, on all days, higher than FEp-Tyr, which remained near-normal, less than 4%. Procalcitonin showed significant correlation with FEm-Tyr (r = 0.454; P < 0.001).
Discussion: Our data suggest that the oxidative stress marker m-Tyr and physiologic p-Tyr may be handled differently in septic patients. The excretion of m-Tyr correlates with inflammation. m-Tyr may be actively secreted or produced in the kidney in some patients, whereas the decreased serum level of p-Tyr is a consequence of diminished renal production and not of renal loss.
Introduction
Inflammation and tissue hypoxia may play a central role in the generation of reactive oxygen species (ROS) in septic patients, and ROS can directly exert renal parenchymal damage and may intensify renal microvascular and functional dysregulation.Citation1–Citation4 Moreover, inflammatory cytokines activate the NADPH oxidase enzyme in the kidney, leading to NADPH oxidase-derived ROS production playing a detrimental role in circulatory system dysfunction and capillary bed plugging.Citation5
We found elevated levels of malondialdehyde (MDA) and myeloperoxidase (MPO) in the early phase of sepsis.Citation6 Production of phorbol-12-myristate-13-acetate-stimulated ROS in whole blood was increased in septic patients on the first and second days of hospitalization.Citation6
Lipids are susceptible targets of oxidation, so products of lipid peroxidation (e.g. MDA) are commonly used markers of oxidative stress. Higher levels of lipid peroxidation products like plasma F2-isoprostane and isofuran were found in septic patients with the development of organ failure.Citation7
Damage to amino acids is mediated by reactive oxygen/nitrogen species. Overproduction of hydroxyl radical converts l-phenylalanine (Phe) into para-, meta-, and ortho-tyrosine (p-, m-, and o-Tyr).Citation8–Citation10 p-Tyr (which is used by other organs for protein synthesis) is formed enzymatically from Phe under physiological circumstances in the kidney. Thus, p-Tyr may be formed physiologically and in oxidative processes, while m- and o-Tyr are oxidative stress markers only. Consequently, elevated levels of m- and o-Tyr refer to hydroxyl radical-induced damage of tissues. Several studies have justified the correlation of m- and o-Tyr levels with other oxidative stress markers.Citation11–Citation13
There was only a small number of clinical studies assessing serum and urinary Tyr isoforms in different pathological conditions. We found lower plasma p-Tyr levels in the chronic kidney disease (CKD) group, and an increased urinary excretion of o-Tyr in diabetic/CKD patients.Citation14 In another study we found that albuminuria (both total and non-immunoreactive) showed a good correlation with urinary o-Tyr/creatinine ratio in patients suffering from ischemic stroke.Citation15 Additionally, we detected higher levels of m-Tyr and o-Tyr in total homogenates of cataractous lenses.Citation16
Hypotheses of the study were as following: (i) The hydroxyl free radical-derived Tyr isoforms are elevated in the sera and urine of septic patients as compared to controls. (ii) Serum levels and urinary excretion of hydroxyl radical-derived Tyr isoforms decrease with amelioration of severity of inflammation in the patients. (iii) Serum levels and kinetics of m- and o-Tyr are determined rather by oxidative stress than by renal handling. (iv) Because of a potential tubular damage, synthesis, and therefore serum levels as well as urinary excretion of the physiological isoform p-Tyr, is lower in septic patients as compared to controls. (v) Despite the potential tubular damage we postulated that p-Tyr still would be effectively retained by the kidneys in most patients. (vi) With the improvement of the patients due to the therapy, p-Tyr levels in the blood of septic patients may normalize. (vii) The renal handling of the structural isomers p-Tyr vs. m- and o-Tyr would be substantially different, despite the minor structural difference.
Materials and methods
Subjects and study design
The study protocol was completed in accordance with the ethical guidelines of the 2003 Declaration of Helsinki. After receiving permission from the Local Ethics Committee (4422/2012), patients or nearest relatives provided a signed informed consent after they were informed clearly about details of the study and blood sampling. This prospective study was performed on 20 patients admitted to our intensive ward between September 2012 and October 2013. Demographic data, Intensive Care Unit (ICU) survival, and source of infections are summarized in Table .
Table 1 Demographic and clinical data of patients
Inclusion criteria were presence of severe sepsis or septic shock at admission. Patients in which sepsis developed as a complication during hospitalization were not featured. Diagnosis of sepsis was based on the American College of Chest Physicians/Society of Critical Care Medicine consensus guidelineCitation17 and positive microbiology cultures.
Medication (e.g. chronic steroid use, immunosuppressive medication) or treatment (e.g. radiotherapy, chemotherapy) affecting immune response, hematologic malignant disease, and oliguria at admission (impossible collection of urine) were all exclusion criteria.
In the overall study period, 75 patients were categorized during the course at ICU as having severe sepsis and, 47 as being in septic shock. Fifty-eight patients met the inclusion criteria (severe sepsis or septic shock at admission). Twenty-six patients were excluded (18 due to oliguria, 6 because of chronic steroid use, and 2 for having malignant diseases). Positive microbiology results were gained in only 20 out of the 32 patients; only these patients were included in the study and they were treated according to the recent sepsis guideline.Citation18 Additionally, 25 healthy individuals were invited to serve as controls for comparison (age: 60 ± 9.15 years; male/female: 7/18, which was not significantly different compared to cases).
Blood samples were taken on admission (day 1) and for four consecutive days (days 2–5) at 7 a.m. Urine was collected every 24 hours and the daily amount was noted. Serum and urinary creatinine levels were measured. To standardize results, urinary levels of the assessed substances were corrected for urinary creatinine, and fractional excretion (FE) was also calculated. FE of a certain substance can be used to examine renal handling of that particular substance. It is calculated by dividing the clearance of the measured substance by the clearance of creatinine. FE shows the amount of the filtered substance excreted with the urine. It therefore indicates whether clearance of the particular substance is greater or smaller than or equal to the clearance of creatinine. If FE of a substance is 100%, it is freely filtered, and the net renal reabsorption and secretion is zero. In case FE is smaller than 100%, it refers to an active renal reabsorption of the substance. If FE exceeds 100%, it indicates active secretion, or in loco renal production of the substance. For calculating 24-hour clearance and FE, the respective blood sample has to be obtained during urine collection.
C-reactive protein (CRP), procalcitonin (PCT), and creatinine levels were measured simultaneously, at the Department of Laboratory Medicine of the University of Pécs.
A 5-day-long study period was chosen because it was presumed that a period of this length would open a time window sufficiently wide for detecting early changes in m-, o-, and p-Tyr levels in patients suffering from sepsis.
Measurement of m-, o-, and p-Tyr levels
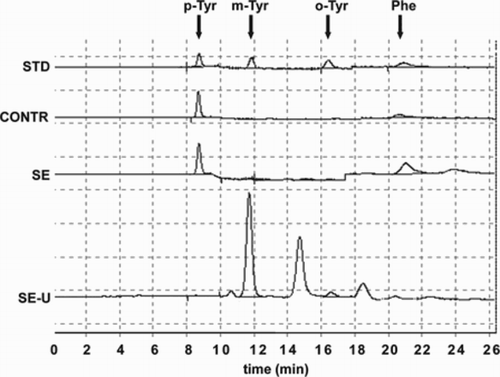
Blood samples of septic patients were obtained from central venous catheter while peripheral blood samples were taken from healthy individuals. Serum was obtained through centrifugation (3000 rpm, 10 minutes). Serum and urine samples were stored at −80°C until further examinations. Serum and urinary m-, o-, p-Tyr and Phe levels were determined using a reverse-phase HPLC method as described earlierCitation14 (Fig. ).
Figure 1 Chromatograms of a standard (STD: 9.375 µmol/l p-Tyr; 187.5 nmol/l m-Tyr; 187.5 nmol/l o-Tyr; 14.062 µmol/l Phe), of sera of a control subject (CONTR), and a septic patient (SE) and chromatogram of a urine sample of a septic patient (SE-U). On the SE-U curve, p-Tyr and Phe show much smaller peaks (at 8.5 and 20.5 minutes, respectively), as they are present in urine in relatively small amounts because of the high rate of their reabsorption. Abbreviations: p-Tyr, para-tyrosine; m-Tyr, meta-tyrosine; o-Tyr, ortho-tyrosine; Phe, phenylalanine.
Statistical analysis
SPSS version 20.0 (IBM Corporation, USA) was used for statistical analysis. Data were expressed as mean ± SD or as median and inter-quartile range (IQR), and depicted on standard box plot. Multiple comparisons (days 1–5) were initiated using the Kruskal–Wallis test, as most parameters were non-normally distributed according to results of the Kolmogorov–Smirnov test. Accordingly, another non-parametric method, the Jonckheere–Terpstra test, was used to detect significant trends. For pairwise comparison of days and groups Student's t-test or Mann–Whitney U test was used, depending on the normal, or non-normal distribution of the variables. Correlational analyses were performed using the non-parametric Spearman's rho test. Values of P < 0.05 were considered as significant.
Results
Serum tyrosine levels
Serum m-Tyr levels did not show significant changes during the study period (using Kruskal–Wallis test or using Jonckheere–Terpstra's test; P = 0.457). Serum m-Tyr levels were significantly higher in septic patients on days 2 (median [IQR]: 16 [38] nM; P = 0.031) and 3 (21 [Citation26] nM; P = 0.035), when compared with those of controls (4 [Citation20] nM) (Fig. A).
Figure 2 Serum levels of (A) m-Tyr, (B) o-Tyr, and (C) p-Tyr in septic patients. Data are expressed as median and IQR (standard twenty-fifth to seventy-fifth percentile and fifth and ninety-fifth confidence interval). Asterisks indicate statistical differences within the septic group compared to day 1 (*: P < 0.05; **: P < 0.01). The ‘#’ symbols show significant differences between patients and controls. (#: P < 0.05; ##: P < 0.01). Serum p-Tyr levels showed a significant day-by-day elevation with trend analysis (P = 0.002).
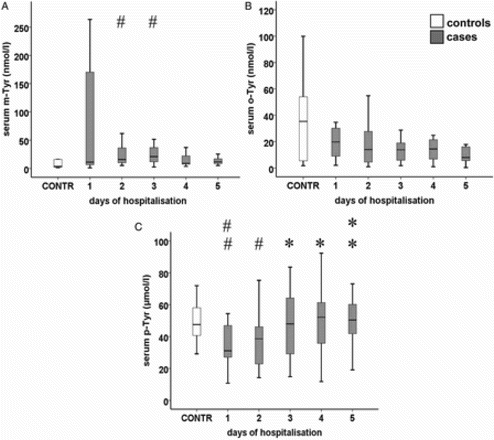
Serum o-Tyr levels did not show significant changes during the study period (P = 0.194). Differences of serum o-Tyr levels between cases and controls were not significant (Fig. B).
Serum p-Tyr levels were lower in septic patients on days 1 (31 [Citation21] µM; P = 0.005) and 2 (39 [Citation26] µM; P = 0.040) as compared to controls. Serum p-Tyr levels showed a day-by-day elevation (P = 0.002), which reached the level of significance on days 3 (48 [39] µM; P = 0.026), 4 (52 [Citation28] µM; P = 0.013), and 5 (50 [Citation28] µM; P = 0.004) compared to day 1, and lead to disappearance of difference compared to controls on days 3–5 (Fig. C).
Urinary tyrosine concentrations
Urinary m-Tyr concentration showed a decreasing tendency (P = 0.041) with a significant difference when comparing day 1 (248 [1651] nM) with day 4 (121 [175] nM; P = 0.029). No significant differences could be observed between cases and controls (data not shown). Urinary o-Tyr concentration did not show any tendency during the study period (P = 0.206), although its level was significantly higher on day 3 (87 [424] nM) when compared to day 1 (575 [3576] nM; P = 0.048) and it was significantly higher in cases (575 [3576] nM, day 1; 255 [1041] nM, day 2; 87 [424] nM, day 3; 107 [616] nM, day 4; 391 [1116] nM, day 5) compared to controls (1 [Citation28] nM) during the whole study period (P < 0.001) (data not shown). Urinary p-Tyr concentrations showed a markedly increasing tendency (P < 0.001) with significant differences on days 3 (27 [73] µM; P = 0.015), 4 (38 [53] µM; P < 0.001), and 5 (45 [78] µM; P < 0.001) when compared with day 1 (11 [Citation16] µM). Its levels were significantly lower on day 1 (P = 0.049) and significantly higher on days 4 and 5 (P = 0.011, P = 0.003, respectively) in cases compared with controls (20 [40] µM; data not shown).
Urinary tyrosine/creatinine ratios
Urinary m-Tyr/creatinine ratios showed a decreasing tendency (P = 0.018) and there was a significant difference between days 1 (63 [542] µM/mM) and 4 (23 [72] µM/mM; P = 0.037). Differences of urinary m-Tyr/creatinine ratios between cases and controls were not significant (Fig. A).
Figure 3 Urinary (A) m-Tyr/creatinine, (B) o-Tyr/creatinine, and (C) p-Tyr/creatinine ratio in septic patients. Data are expressed as median and IQR ((standard twenty-fifth to seventy-fifth percentile and fifth and ninety-fifth confidence interval). Asterisks indicate statistical differences within the septic group compared to day 1 (*: P < 0.05; **: P < 0.01; ***: P < 0.001). The ‘#’ symbols show significant differences between patients and controls. (#: P < 0.05; ##: P < 0.01; ###: P < 0.001). Urinary m-Tyr/creatinine ratios had a decreasing tendency (P = 0.018), while urinary p-Tyr/creatinine ratios showed a marked increase (P = 0.001).
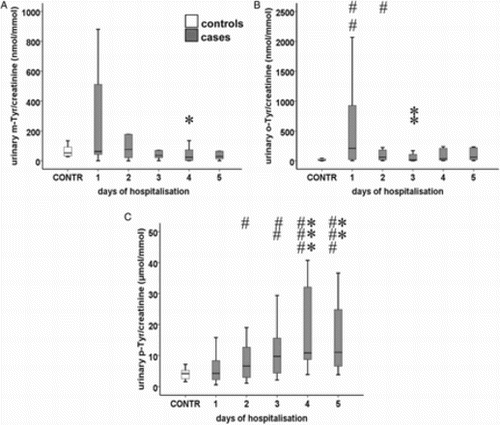
Urinary o-Tyr/creatinine ratios did not show any tendency during the study period; o their data were significantly higher in cases than in controls (12 [35] µM/mM) on days 1 (209 [950] µM/mM) and 2 (60 [177] µM/mM; P = 0.009 and P = 0.041, respectively). o-Tyr/creatinine ratios were significantly lower on day 3 (16 [136] µM/mM) vs. day 1 (P = 0.009) (Fig. B).
Urinary p-Tyr/creatinine ratios showed a marked increase (P = 0.001), with significant differences on days 4 (11 [Citation24] µM/mM; P = 0.001) and 5 (11 [Citation25] µM/mM; P = 0.006) compared to day 1 (4 [Citation6] µM/mM). Urinary p-Tyr/creatinine ratios were higher in septic patients on days 2 (7 [Citation10] µM/mM; P = 0.037), 3 (10 [Citation12] µM/mM; P = 0.006), 4 (P < 0.001) and 5 (P < 0.001) compared to those of controls (4 [Citation3] µM/mM) (Fig. C).
FE of the tyrosine isomers
FE of m-Tyr (FEm-Tyr) showed a decreasing tendency (P = 0.009) and on day 5 (25.0 [50.9] %) a significant difference could be observed compared to day 1 (61.0 [149.0] %; P = 0.023), while FEo-Tyr and FEp-Tyr remained unchanged (Fig. A and B). FEp-Tyr was higher on day 4 (2.8 [4.9] %) than on day 1 (1.6 [1.7] %; P = 0.031).
Figure 4 (A) FE of p-, m-, and o-Tyr (IQR; standard twenty-fifth to seventy-fifth percentile and fifth and ninety-fifth confidence interval). FE of m-Tyr showed a decreasing tendency (P = 0.009). (B) FE of p-Tyr (IQR; standard twenty-fifth to seventy-fifth percentile and fifth and ninety-fifth confidence interval). Asterisk indicates a statistically relevant difference within the septic group compared to day 1 (*: P < 0.05). The ‘#’ symbols show significant differences between cases and controls (#: P < 0.05; ##: P < 0.01; ###: P < 0.001). The ‘+’ symbols show significant differences between FE of m- or o-Tyr and that of p-Tyr (+: P < 0.05; ++: P < 0.01; +++: P < 0.001).
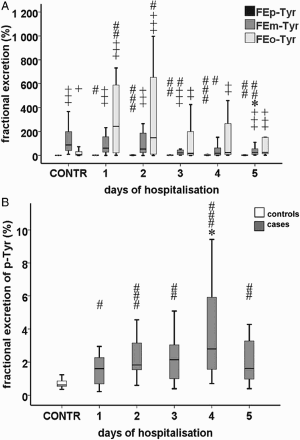
FEm-Tyr values in cases were significantly lower on days 3 (21.8 [42.5] %), 4 (18.7 [64.8] %), and 5 (P = 0.030; P = 0.026; P = 0.009, respectively) than those of controls (87.5 [171.3] %). FEo-Tyr was significantly higher in cases on days 1 (243.0 [598.6] %) and 2 (147.9 [693.2] %; P = 0.006; P = 0.020, respectively) in comparison with controls (5.2 [37.6] %) (Fig. A). FEp-Tyr levels were significantly higher in cases than in controls (0.6 [0.4] %) during the whole study period (1.6 [1.7] %; P = 0.027, day 1; 1.8 [1.8] %; P < 0.001, day 2; 2.1 [2.5] %; P = 0.007, day 3; 2.8 [4.9] %; P < 0.001, day 4; 1.6 [2.4] %; P = 0.003, day 5) (Fig. B).
FEm-Tyr was significantly higher than FEp-Tyr throughout almost the whole study period (61.0 [149.0] vs. 1.6 [1.7] %; P < 0.001, day 1; 53.6 [217.7] vs. 1.8 [1.8] %; P < 0.001, day 2; 21.8 [42.5] vs. 2.1 [2.5] %; P < 0.001, day 3; 18.7 [64.8] vs. 2.8 [4.9] %; P = 0.054, day 4; 25.0 [50.9] vs. 1.6 [2.4] %; P < 0.001, day 5, respectively). FEo-Tyr was significantly higher than FEp-Tyr almost throughout the entire study period (243.0 [598.6] vs. 1.6 [1.7] %; P < 0.001, day 1; 147.9 [693.2] vs. 1.8 [1.8] %; P < 0.001, day 2; 17.4 [205.8] vs. 2.1 [2.5] %; P = 0.007, day 3; 23.7 [306.7] vs. 2.8 [4.9]; P = 0.002, day 4; 25.7 [205.2] vs. 1.6 [2.4] %; P < 0.001, day 5, respectively). Interestingly, FEm-Tyr (87.5 [171.3] %) and FEo-Tyr(5.2 [37.6] %) were also significantly higher compared to FEp-Tyr (0.6 [0.4] %; P < 0.001 and P = 0.019, respectively) in the case of controls. On days 1 and 2, 6 out of 20 cases (30%) had an FEm-Tyr value above 100%, while all FEp-Tyr values remained less than 4% throughout the study.
Although we found no significant differences between survivor and non-survivor patients regarding FEm-Tyr, FEo-Tyr, or FEp-Tyr on day 1, a notable difference in FEo-Tyr between survivor and non-survivor groups was observed as near significant (P = 0.057) (Table ).
Table 2 Clinical data of patients on day 1
Clinical parameters and their correlation with tyrosine values
Serum CRP levels showed a decreasing tendency as judged by Jonckheere–Terpstra's test (P = 0.015). Assessing data of the entire study period, serum levels of p-Tyr (r = −0.536; P < 0.001), Phe (r = −0.266; P = 0.019), and serum p-Tyr/Phe ratio (r = −0.284; P = 0.012) showed negative correlation with CRP. Serum m-Tyr/p-Tyr (r = 0.267; P = 0.021) and o-Tyr/p-Tyr ratios (r = 0.268; P = 0.019) showed positive correlation with CRP (data not shown).
Serum PCT levels showed a decreasing tendency (P = 0.027) with the highest level on day 2 (Fig. A). Assessing the data of the entire study period, urinary m-Tyr concentration (r = 0.302; P < 0.001), urinary m-Tyr/creatinine ratio (r = 0.319; P < 0.001), and FEm-Tyr (r = 0.454; P < 0.001) showed a positive correlation with PCT. Urinary p-Tyr concentration (r = −0.291; P = 0.001) and urinary p-Tyr/creatinine ratio (r = −0.202; P = 0.028) showed a negative correlation with PCT (data not shown).
Figure 5 Serum levels of (A) PCT and (B) creatinine in septic patients. Data are expressed as median and IQR (standard twenty-fifth to seventy-fifth percentile and fifth and ninety-fifth confidence interval). A significant decreasing tendency was found in both parameters (P = 0.027; P = 0.033, respectively).
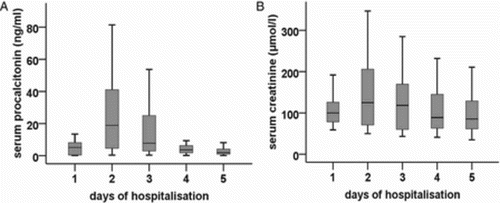
Serum creatinine showed a decreasing tendency (P = 0.033), with the highest level on day 2 (Fig. B). Its levels correlated with urinary m-Tyr concentration (r = 0.178; P = 0.031), urinary m-Tyr/creatinine ratio (r = 0.232; P = 0.004), urinary o-Tyr/creatinine ratio (r = 0.166; P = 0.046), FEm-Tyr (r = 0.411; P < 0.001), FEo-Tyr (r = 0.361; P < 0.001), urinary p-Tyr concentration (r = −0.514; P < 0.001), and p-Tyr/creatinine ratio (r = −0.416; P < 0.001) (data not shown).
Furthermore, comparing survivor and non-survivor patients, we found significant differences in urine production on day 1 (P = 0.006). Regarding all the other clinical parameters, we did not find any significant differences when comparing survivors and non-survivors on day 1 (Table ).
Potential confounder role of antibiotic therapy
Patients regarding their antibiotic treatment have been compared. As most of the subjects are given a combination of different antibiotic medications, we performed a comparison concerning, whether or not a patient has received antibiotics of a certain class (penicillins and cephalosporins, aminoglycosides, fluoroquinolones, macrolides, glycopeptides, carbapenems, polymyxines, or tetracyclines). In case of three classes of antibiotics, we found interesting differences in Tyr parameters. FEp-Tyr was significantly lower in patients given penicillin or cephalosporin treatment (1.1 [1.4] %) as compared to those not receiving these drugs (2.2 [2.0] %; P = 0.003). Similarly, subjects with aminoglycoside treatment had lower FEp-Tyr (1.6 [1.9] %) than subjects not receiving aminoglycosides (2.3 [2.5] %; P = 0.015). Finally, patients with carbapenem treatment had lower serum level of p-Tyr (31 [Citation22] µM) and higher FEo-Tyr (158.5 [432.9] %) than subjects without carbapenem treatment (50 [Citation22] µM; P < 0.001, 22.6 [243.5] %; P = 0.043, respectively). We did not find any difference in Tyr parameters regarding administration of fluoroquinolones (data not shown). There were only a few patients given macrolide, glycopeptide, polymyxine, or tetracycline treatment, in which cases statistical comparison was impossible.
Discussion
The main findings of our study were that serum levels of m-Tyr were significantly higher in septic patients on days 2 and 3 than in those of the controls. Urinary m-Tyr/creatinine ratios and also FEm-Tyr showed a significant tendency of decrease during the study period. Serum levels of p-Tyr were lower in septic patients on days 1 and 2 than in those of controls and this showed a significant day-by-day elevation, which led to a disappearance of the differences between cases and controls on days 3–5. Urinary p-Tyr/creatinine ratios increased during the 5 days of our observation. The level of FEp-Tyr was significantly lower than that of FEm-Tyr during the whole study period. Measured tyrosines showed correlations with relevant clinical parameters of inflammation and renal function.
In the human body, three isoforms of l-tyrosine can be detected, namely m-, o-, and p-Tyr. The earlier two (m- and o-Tyr) are mainly formed non-enzymatically due to the attack by hydroxyl free radical on Phe molecules, while the latter (p-Tyr) is mainly formed in enzymatic reactions (by phenylalanine hydroxylase (PAH)) from Phe.Citation8,Citation19
We found higher serum m-Tyr levels in septic patients compared with controls on days 2 and 3. A significant decreasing tendency in serum m-Tyr has led to the disappearance of differences between cases and controls on days 4 and 5. Additionally, serum PCT levels (the marker of bacterial infection)Citation20 have shown a significant decreasing tendency in parallel with the decrease of serum m-Tyr levels. Similar to our results, several other studies have shown similar tendencies regarding PCT, MDA, and MPO in case of adequate antibiotic treatment.Citation6,Citation21,Citation22
Another factor that might have an influence on initial serum m-Tyr levels and their subsequent changes might be impaired renal function, because serum creatinine levels changed in parallel with serum m-Tyr levels, and renal injury has been shown to induce oxidative stress.Citation23 A further possible explanation of the initially high serum levels of m-Tyr (as compared to controls) might be an initial low renal clearance leading to its accumulation, which might improve alongside the amelioration of renal function, and could eventually also lead to the normalization of m-Tyr levels.
We found decreasing urinary m-Tyr/creatinine ratios throughout the study. A decrease in the urinary excretion of a substance can be explained by (i) decreasing serum levels with the same rate of renal transport, (ii) normal serum levels and decreasing rate of renal transport, or (iii) combination of decreasing serum levels and decreasing rates of renal transport. In order to gain more information on renal transport of m-Tyr, FE of m-Tyr was also analyzed. A substantial proportion (30%) of patients had FEm-Tyr levels above 100% at the beginning of the study. This suggests that in these patients m-Tyr is either secreted actively by the kidney, or is produced in loco in the kidney, due to hydroxyl free radical attack. This is also supported by the correlation between PCT levels and the urinary m-Tyr/creatinine ratio or FEm-Tyr. FEm-Tyr showed a decreasing tendency during the study, which was also in line with a decrease in inflammation resulting from therapy.
Furthermore, our data suggest that initial high serum levels of m-Tyr (as compared to controls) do not occur as a result of renal retention of m-Tyr (in this case FEm-Tyr should be <100% in all cases), but rather to a systemic overproduction of the substance due to oxidative stress processes. Based on these findings, we can also hypothesize that the decreasing tendency of the urinary concentration of m-Tyr occurs due to a combination of the continuously decreasing serum m-Tyr levels and decreased urinary clearance independent of the glomerular filtration rate as suggested by a decrease in FEm-Tyr (i.e. possibility (iii), see above).
As to o-Tyr, no significant tendency was observed during the course of the study for urinary o-Tyr/creatinine levels or FEo-Tyr. However, FEo-Tyr exceeded 100% in 50, 35, 30, 25, and 25% of our cases on days 1–5, respectively. This result suggests that o-Tyr is actively excreted, or produced in loco in the kidney. This is in line with our previous findings in other patient populations, namely in patients with type 2 diabetes mellitus ± CKDCitation14 and in patients with hypertension ± type 2 diabetes mellitus ± CKD, where we have found patients having FEo-Tyr >100% in up to 30% of the cases (partially unpublished data).Citation24 Different behavior of m- and o-Tyr is an interesting finding of our study, supported by the data of others as well, an explanation of which is unknown as yet.Citation9
Regarding p-Tyr, serum levels of the physiological amino acid were lower at the beginning of the study compared to those of controls. During the study period, serum p-Tyr showed an increasing tendency, which finally led to the normalization of p-Tyr levels. Potential explanations for initial low serum p-Tyr levels in septic patients might be the following: (i) a catabolic condition that leads to lower levels of amino acids, (ii) impaired renal synthesis of p-Tyr, or (iii) increased renal loss due to lack of proximal tubular reabsorption. Amelioration of any of the above mentioned three processes could explain normalization of p-Tyr serum levels.
| (i) | In fact, a catabolic state can be found in sepsis due to increased levels of pro-inflammatory cytokines and stress hormones.Citation25 However, in our study we found that serum Phe levels of our patients were not lower than those of controls (data not shown), thereby excluding the possibility that a general amino acid metabolic problem due to a catabolic state could be, per se, responsible for the lower serum levels of p-Tyr. In order to obtain further information about the other two possible options, urinary excretion and FE of p-Tyr needed to be analyzed. | ||||
| (ii) | Low serum p-Tyr levels due to low PAH activity are mainly known from patients with severe impairment of glomerular function, including those on hemodialysis. In our study, no correlation could be found between serum creatinine levels and serum p-Tyr levels (data not shown), which suggests that in this phase of renal damage, lower p-Tyr levels could occur without severe impairment of glomerular function. Another explanation for low PAH activity and resulting low p-Tyr level could be deficiency of the cofactor used in the enzymatic reaction. In fact, several studies reported higher levels of Phe in septic patients,Citation26–Citation28 moreover maximum values of Phe were significantly associated with mortality in patients with sepsis.Citation29 The underlying cause of elevated serum Phe levels may indeed be the decreased PAH activity. Namely, as a result of oxidative stress, the enzyme cofactor tetrahydrobiopterin (BH4) is oxidized non-enzymatically to dihydrobiopterin.Citation30 Administration of BH4 could improve microcirculation and outcome in an ovine sepsis model.Citation31 Although there was a tendency of higher Phe levels, this did not prove to be significant. It can be explained by the relatively low number of cases, or by the fact that our patients were hypoalbuminemic (median [IQR]: 25.7 [6.9] g/l for whole study period, 26.9 [5.4] g/l maximum on day 3) and hypoproteinemic (42.8 [12.9] g/l for whole study period, 44.0 [21.2] g/l maximum on day 1) (data not shown). Therefore, excessive amounts of Phe would be used up in protein synthesis. Nevertheless, the potential role of BH4 deficiency is also feasible. | ||||
| (iii) | FEp-Tyr remained well below 100% throughout the study, but it was higher than the 1% suggested by the literatureCitation32 or our earlier data on controls,Citation14 indicating a slight impairment of renal tubular reabsorption, which could in fact influence serum p-Tyr levels. Another possibility could be active secretion of in loco produced p-Tyr into the tubular lumen.Citation33 | ||||
Antibiotic treatment might have an effect on renal function, including tubular function (e.g. aminoglycosides may be directly nephrotoxic), and it could also have an influence on amino acid transport and metabolism. To investigate this issue, we compared patients regarding their antibiotic treatment. We found that use of beta-lactams, aminoglycosides, and carbapenem had an effect on Tyr parameters. This connection could be described by different approaches, e.g. potential tubular effect of these agents, or role of the underlying primary disease, which determines both antibiotic treatment, and which could also have an effect on amino acid metabolism, transport, etc. Thus, the confounder role of these medications cannot be excluded. The exact effect of the antibiotics would need further analyses that are out of scope of this study.
Limitations of our study are: (i) the number of patients was too low to allow us to draw negative conclusions concerning the tendencies of the measured parameters. As indicated in the title, this is a pilot study; (ii) previous data on the distinct isomers do not exist in sepsis. The necessary number of cases for a future study has to be calculated based on these data.
The striking differences between FEm-Tyr and FEp-Tyr may indicate that the two amino acids (p-Tyr and m-Tyr) showing only minor structural differences are handled by the kidneys in a completely different way in septic patients.
Acknowledgments
The research of G. A. Molnar was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4. A/2-11-1-2012-0001 ‘National Excellence Program’.
Disclaimer statements
Contributors
Funding
Conflicts of interest The authors have declared that no conflicts of interest exist.
Ethics approval The study protocol was completed in accordance with ethical guidelines of the 2003 Declaration of Helsinki and it was approved by the Local Ethics Committee (4422/2012).
References
- Crimi E, Sica V, Slutsky AS, Zhang H, Williams-Ignarro S, Ignarro LJ, et al. Role of oxidative stress in experimental sepsis and multisystem organ dysfunction. Free Radic Res 2006;40:665–72. doi: 10.1080/10715760600669612
- Wheeler DS. Oxidative stress in critically ill children with sepsis. Open Inflamm J 2011;4:74–81. doi: 10.2174/1875041901104010074
- Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 2013;1:483–91. doi: 10.1016/j.redox.2013.07.006
- Heyman SN, Rosen S, Rosenberger C. A role for oxidative stress. Contrib Nephrol 2011;174:138–48. doi: 10.1159/000329383
- Tyml K. Critical role for oxidative stress, platelets, and coagulation in capillary blood flow impairment in sepsis. Microcirculation 2011;18:152–62. doi: 10.1111/j.1549-8719.2010.00080.x
- Mühl D, Woth G, Drenkovics L, Varga A, Ghosh S, Csontos C, et al. Comparison of oxidative stress and leukocyte activation in patients with severe sepsis and burn injury. Indian J Med Res 2011;134:69–78.
- Ware LB, Fessel JP, May AK, Roberts LJ2nd. Plasma biomarkers of oxidant stress and development of organ failure in severe sepsis. Shock 2011;36:12–7. doi: 10.1097/SHK.0b013e318217025a
- Stadtman ER, Berlett BS. Fenton chemistry – amino acid oxidation. J Biol Chem 1991;266:17201–11.
- Galano A, Cruz-Torres A. HO radical reactions with phenylalanine in free and peptide forms. Org Biomol Chem 2008;6:732–8. doi: 10.1039/b716024k
- Karam LR, Simic MG. Formation of ortho-tyrosine by radiation and organic solvents in chicken tissue. J Biol Chem 1990;265:11581–5.
- Lubec B, Hayn M, Denk W, Bauer G. Brain lipid peroxidation and hydroxyl radical attack following the intravenous infusion of hydrogen peroxide in an infant. Free Radic Biol Med 1996;21:219–23. doi: 10.1016/0891-5849(96)00018-4
- Dandona P, Mohanty P, Hamouda W, Ghanim H, Aljada A, Garg R, et al. Inhibitory effect of two day fast on reactive oxygen species (ROS) generation by leukocytes and plasma ortho-tyrosine, meta-tyrosine concentrations. J Clin Endocrinol Metab 2001;86:2899–902. doi: 10.1210/jcem.86.6.7745
- Jörres RA, Holz O, Zachgo W, Timm P, Koschyk S, Müller B, et al. The effect of repeated ozone exposures on inflammatory markers in bronchoalveolar lavage fluid and mucosal biopsies. Am J Respir Crit Care Med 2000;161:1855–61. doi: 10.1164/ajrccm.161.6.9908102
- Molnár GA, Wagner Z, Markó L, Kőszegi T, Mohás M, Kocsis B, et al. Urinary ortho-tyrosine excretion in diabetes mellitus and renal failure: evidence for hydroxyl radical production. Kidney Int 2005;68:2281–7. doi: 10.1111/j.1523-1755.2005.00687.x
- Toth P, Koller A, Pusch G, Bosnyak E, Szapary L, Komoly S, et al. Microalbuminuria, indicated by total versus immunoreactive urinary albumins, in acute ischemic stroke patients. J Stroke Cerebrovasc Dis 2011;20:510–6. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.002
- Molnár GA, Nemes V, Biró Z, Ludány A, Wagner Z, Wittmann I. Accumulation of the hydroxyl free radical markers meta-, ortho-tyrosine and DOPA in cataractous lenses is accompanied by a lower protein and phenylalanine content of the water-soluble phase. Free Radic Res 2005;39:1359–66. doi: 10.1080/10715760500307107
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock 2012. Intensive Care Med 2013;39:165–228. doi: 10.1007/s00134-012-2769-8
- Stadtman ER, Levine ER. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003;25:207–18. doi: 10.1007/s00726-003-0011-2
- Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993;341:515–8. doi: 10.1016/0140-6736(93)90277-N
- Charles PE, Tinel C, Barbar S, Aho S, Prin S, Doise JM, et al. Procalcitonin kinetics within the first days of sepsis: relationship with the appropriateness of antibiotic therapy and the outcome. Crit Care 2009;13:R38. doi: 10.1186/cc7751
- Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA. Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med 2000;28:2591–4. doi: 10.1097/00003246-200007000-00068
- Qian J, You H, Zhu Q, Ma S, Zhou Y, Zheng Y, et al. Nitrotyrosine level was associated with mortality in patients with acute kidney injury. PLoS One 2013;8:e79962. doi: 10.1371/journal.pone.0079962
- Nagy G, Gaszner B, Lányi É, Markó L, Fehér E, Cseh J, et al. Selective association of endogenous ouabain with subclinical organ damage in treated hypertensive patients. J Hum Hypertens 2011;25:122–9. doi: 10.1038/jhh.2010.24
- Biolo G. Protein metabolism and requirements. World Rev Nutr Diet 2013;105:12–20. doi: 10.1159/000341545
- Ploder M, Neurauter G, Spittler A, Schroecksnadel K, Roth E, Fuchs D. Serum phenylalanine in patients post trauma and with sepsis correlate to neopterin concentrations. Amino Acids 2008;35:303–7. doi: 10.1007/s00726-007-0625-x
- Ollenschlager G, Jansen S, Schindler J, Rasokat H, Schrappe-Bacher M, Roth E. Plasma amino acid pattern of patients with HIV infection. Clin Chem 1988;34:1787–9.
- Roth E, Zoch G, Schulz F, Karner J, Muhlbacher F, Hamilton G, et al. Amino acid concentrations in plasma and skeletal muscle of patients with acute hemorrhagic necrotizing pancreatitis. Clin Chem 1985;31:1305–9.
- Hirose T, Shimizu K, Ogura H, Tasaki O, Hamasaki T, Yamano S, et al. Altered balance of the aminogram in patients with sepsis – the relation to mortality. Clin Nutr 2014;33:179–82. doi: 10.1016/j.clnu.2013.11.017
- Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Weinberg JB, et al. Impaired systemic tetrahydrobiopterin bioavailability and increased dihydrobiopterin in adult falciparum malaria: association with disease severity, impaired microvascular function and increased endothelial activation. PLoS Pathog 2015;11:e1004667. doi: 10.1371/journal.ppat.1004667
- He X, Su F, Velissaris D, Salgado DR, de Souza Barros D, Lorent S, et al. Administration of tetrahydrobiopterin improves the microcirculation and outcome in an ovine model of septic shock. Crit Care Med 2012;10:2833–40. doi: 10.1097/CCM.0b013e31825b88ba
- Bergeron M, Scriver CR. Pathophysiology of renal hyperaminoacidurias and glucosuria. In: Seldin DW, Giebisch G, (eds.) The kidney. Physiology and pathophysiology. New York: Raven Press; 1985. pp. 1725–45.
- Wang Y, DeMayo JL, Hahn TM, Finegold MJ, Konecki DS, Lichter-Konecki U, et al. Tissue- and development-specific expression of the human phenylalanine hydroxylase/chloramphenicol acetyltransferase fusion gene in transgenic mice. J Biol Chem 1992;267:15105–10.
