Abstract
Objectives:
Our aim is to describe the impact of emtricitabine (FTC)/tenofovir (TDF) versus other nucleoside reverse transcriptase inhibitor (NRTIs)-based regimens on renal function of human immunodeficiency virus (HIV) naïve patients >50 years old who started combination antiretroviral therapy (cART).
Design:
National, retrospective cohort analysis of patients >50 years old when they started cART (January 1, 2006–December 31, 2009).
Methods:
We compared renal safety (changes in estimated glomerular filtration rate [eGFR] during the first year, and time to renal events during 4 years of follow-up) in FTC/TDF versus non-FTC/TDF users. Among FTC/TDF users, we compared protease inhibitors vs non-nucleoside reverse transcriptase inhibitors and Lopinavir/ritonavir vs Efavirenz.
Results:
We included 103 patients: median age: 54.9 years, 84% males, median CD4 count 247 cells/μl, median viral load 4.7 log; median follow up 18 months (max: 48 months); 73 started with FTC/TDF and 30 with other NRTIs. Change in eGFR was significantly worse for ritonavir-boosted lopinavir (LPV/r) vs efavirenz (EFV) users in the FTC/TDF group (71.2 vs 98.9 ml/min/1.73 m2 at month 12, P < 0.05). The risk of renal events (progression to an Chronic Kidney Disease Epidemiology Collaboration value < 60 ml/min/1.73 m2 in subjects with baseline values >60) was comparable for FTC/TDF users and non users, but was higher and almost significant for LPV/r as compared to EFV users in the FTC/TDF group (adjusted hazard ratio 6.1, 95% CI 0.8–45.5).
Conclusions:
In our study with a population of HIV infected subjects ≥ 50 years old, renal safety was similar for FTC/TDF and other NRTI-based regimens, but worse for LPV/r as compared to other regimens.
Data Partially Presented at: Eleventh International Congress on Drug Therapy in HIV Infection, 11-15 November 2012, Glasgow, UK. Poster P047 IV Congreso Nacional GESIDA. Toledo, Spain, November 2012. Poster P050.
Introduction
Tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) have a potent activity against the human immunodeficiency virus (HIV), and are nowadays one of the cornerstones of combined antiretroviral therapy (cART).Citation1 Although TDF is usually well tolerated and safe,Citation2,Citation3 some patients develop nephrotoxicity related to its use, leading to approximately 1–2% discontinuation in clinical trials.Citation4–Citation6 This nephrotoxicity is usually evident as a tubular dysfunctionCitation5,Citation7 or as other forms of a TDF-induced renal impairment.Citation2,Citation8 The debate about the renal safety of TDF is especially important in older patients; in this group, special caution has been suggested for the use of TDF.Citation9 However, potential confounders as the presence of comorbidities and other therapies not related to HIV-infection, more frequent among older patients, could be implicated in these findings.Citation10,Citation11
We performed a retrospective study with the aim to describe the impact of FTC/TDF versus other nucleoside reverse transcriptase inhibitors (NRTIs)-based regimens on renal function of HIV naïve patients who started ART being 50 years old or more.
Methods
The TRIP study is a national, retrospective cohort analysis of HIV-infected patients aged 50 years old or more at the time they began their first cART regimen, and has been described elsewhere.Citation12 Briefly, eligible subjects had to have initiated their first cART between January 1, 2006 and December 31, 2009 in the HIV units of 18 hospitals across Spain. All consecutive subjects who met inclusion criteria during the study period were invited to participate; the only exclusion criterion was to have participated in a clinical trial during the study period. Patients had received their treatments (antiretroviral and others) according to their physician's decision, and were classified as having an FTC/TDF regimen if these drugs were included in their first regimen (FTC/TDF group), or a non-FTC/TDF regimen if they were not (non-FTC/TDF group). Among FTC/TDF users, they were also classified according to the third agent as receiving a protease inhibitors (PI; boosted or not, PI group) or a non-nucleoside reverse transcriptase inhibitors (NNRTI; NNRTI group), and also as receiving ritonavir boosted lopinavir (LPV/r group) or efavirenz (EFV group). The study was designed to recruit subjects in a proportion 2:1 to FTC/TDF vs non-TDF regimens, respectively.
All data were retrospectively obtained from the medical records. Data collected included information on sociodemographics: age at the beginning of cART, gender, self-reported place of birth, HIV transmission category, time since HIV diagnosis and CDC stage; and anthropometric measurements including weight, height and blood pressure. Laboratory measurements at baseline and during follow up included, among others, serum creatinine, estimated glomerular filtration rate (eGFR) according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation,Citation13 and urinary sediment (presence or absence of any pathological finding, glucosuria and/or hematuria). CKD-EPI eGFR was considered abnormal if it was < 60 ml/min/1.73 m2. Information about the presence of concurrent comorbidities and concomitant medications was collected, and those subjects who were taking drugs considered as potentially nephrotoxic (pentamidine, trimethoprim/sulfamethoxazole, ibuprofen, oxaliplatin, and pegylated interferon) were excluded from this analysis.Citation7,Citation14,Citation15
Renal endpoints were defined as changes from baseline to week 48 in the glomerular filtration rate (CKD-EPI formula), and time to renal events during follow-up, censored at 48 months. Renal event was defined as progression to an eGFR < 60 ml/min/m2 in subjects with baseline values ≥ 60.
Statistical analysis
Descriptive analyses of demographic characteristics and clinical parameters were made using frequency distributions or median and interquartile ranges; differences by initial regimen were tested through chi-square or U Mann–Whitney tests. Time to renal events was assessed by survival analyses (Kaplan–Meier curves with log rank tests and Cox regression models adjusted by the potential confounders) considering the observation period between January 1, 2006 and September 30, 2011, and censored at 48 months or by the time of the first change in any component of the initial regimen.
Results
During the study period a total of 161 patients were included in the TRIP study. Of them, 58 were receiving potentially nephrotoxic drugs at baseline or had incomplete information about renal function, and were excluded from this analysis. Baseline characteristics of the 103 subjects included are described in . Seventy-three subjects started with FTC/TDF and 30 with other NRTIs (non-FTC/TDF); baseline characteristics were similar between groups, except for the distribution by transmission category. Median time of follow-up was 18 months.
Table 1. Baseline characteristics, stratified by baseline regimen
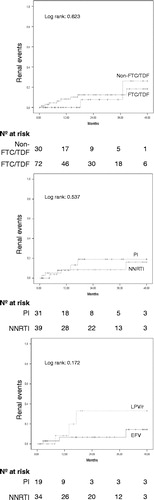
At baseline, median eGFR was similar for FTC/TDF users and non-users, and for PI/NNRTI or lopinavir (LPV)/efavirenz (EFV) users. At month 12 eGFR was higher for non-FTC/TDF users than for users, due to an increase in the non-users group, with stable values for users. Among FTC/TDF users, those who received LPV/r experienced a decrease of almost 18 ml/min/1.73 m2, and the median value at this time point was significantly lower for them as compared to EFV users ().
Table 2. Evolution of glomerular filtration rate (CKD-EPI), and proportion and risk of renal events
also shows the proportion and risk of renal events. During 48 months of follow-up, 10/103 patients (10%) presented with renal events. Differences between FTC/TDF users and non-users were not statistically significant (11.1 vs 6.7%; log rank 0.623), but risk for LPV as compared to EFV among FTC/TDF users was higher and almost significant (log rank 0.172; adjusted hazard ratio: 6.15; 95% confidence interval [95% CI]: 0.83, 45.48) ( and ). The global Cox regression model for this endpoint shows, in the univariate analysis, that the use of FTC/TDF is not associated with renal risk (HR = 1.47, 95% CI 0.31–6.94, P = 0.625), and age implies an increasing risk with each increasing year (HR = 1.07, 95% CI 1.003–1.14, P = 0.039). The multivariate models cold not identify any factor associated to this risk.
Discussion
This study has shown that the FTC/TDF backbone is a safe option in this population of HIV-infected patients who start ART being 50 years old or more, not exposed to other known nephrotoxic agents and with a preserved renal function, especially if it is not combined with LPV/r.
In our study, renal safety was similar when FTC/TDF was compared to other NRTIs regimens. Previous studies have linked a higher risk of chronic kidney failure when TDF was associated with some ritonavir boosted PIs (i.e. atazanavir, lopinavir, saquinavir).Citation9,Citation16 Although some authors linked it to the cumulative exposure to TDF,Citation7,Citation17 suggesting that its long-term use increased the risk of nephrotoxicity,Citation18 after more than 12 months of follow-up we have not observed important adverse events related to the eGFR.
Similar to Young et al.,Citation19 we observed that first regimens that combine TDF with boosted PIs, such as LPV/r, lead to a decrease in eGFR. Other studies show differences at 48 weeksCitation20–Citation22 that are not always statistically significant;Citation22 however the decline in eGFR is always greater when TDF is co-administrated with boosted PIs. One of the possible mechanisms could be that the TDF maximum concentration and the concentration at the end of the dosing interval are 15 and 51% higher, respectively, when TDF was coadministered with LPV/r.Citation23 This does not mean that the use of TDF plus boosted PIs, if necessary, is contraindicated in this population, but means that it requires a more comprehensive analysis of renal function while these regimens are being used.
Previous studies have suggested older age, more advanced HIV infection, lower Body Mass Index, impaired renal function and the coadministration of PIs or additional nephrotoxic drugs as possible risk factors for TDF nephrotoxicity.Citation4,Citation21,Citation24,Citation25. In the series of Lubomirov et al.,Citation26 14% of the patients on TDF treatment discontinued this antiretroviral in the first year of treatment. The final Cox analysis identified the body weight < 60 kg (HR = 2.71; P 0.024) or between 60 and 69 kg (HR = 2.43; P 0.024) as a significant variable related to the TDF discontinuation. Similar findings were reported by Nishijima et al.,Citation27 where the incidence of renal dysfunction in patients ≤ 60 kg treated with TDF was twice as higher as those treated with abacavir. In our series, baseline weight was not statistically different between groups.
There are limitations in our study. First, this is an observational study, although we think we have minimized possible confounding factors as we have considered the presence of comorbidities and use of nephrotoxic drugs. However it is possible that the use of TDF may have been avoided in many patients with baseline renal insufficiency. Second, the study is also underpowered for drawing conclusions about some of the antiretroviral regimens. However, to our knowledge, this is the first study that has evaluated the renal impact of cART in elderly HIV-infected patients, and has included a high number of patients with a long follow-up of about 4 years.
In conclusion, in an increasingly ageing HIV-infected population, our study contributes to the knowledge of cART functioning in these patients, supporting the evidence of renal safety of the FTC/TDF fixed-dose combination in ageing patients who start their first cART, and showing a worse profile for LPV/r as compared to other regimens, when combined with FTC/TDF. In the absence of randomized clinical trials, our data may contribute to a better understanding of the safety of cART in this population.
Disclaimer statements
Contributors
EP, AMCM, PF and JRB initiated this project. All authors were responsible for data collection. AMCM performed the analysis. EP, AMCM and JRB drafted the manuscript. All the authors involved in the study revised the manuscript for important intellectual content and contributed to the final version of the manuscript.
Funding
This study was supported by an unrestricted scientific grant from Gilead Sciences.
Conflicts of Interest
Dr. Pedrol reports grants from Gilead Sciences, during the conduct of the study; other from Janssen, outside the submitted work. Dr. Caro-Murillo is employed by Michael Page Interim Management doing full-time support to the medical department of Gilead Sciences SL. Dr. Castaño has nothing to disclose. Dr Riera reports personal fees from Abbie, BMS, Jansen, Merk and Gilead outside the submitted work. Dr. Olalla reports personal fees from Gilead, MSD, Abbie and ViiV, outside the submitted work. Dr. Domingo has nothing to disclose. Dr. Arazo has nothing to disclose. Dr. Gomez-Sirvent reports personal fees and non-financial support from Gilead Laboratories, and personal fees from Janssen Laboratories, ViiV Laboratories, Abbie Laboratories and Merck Laboratories, outside the submitted work. Dr. Pulido reports grants from Gilead, during the conduct of the study; personal fees from Abbvie, personal fees from BMS, personal fees from Gilead, personal fees from Janssen, personal fees from MSD, personal fees from Viiv, outside the submitted work. Dr. Romero has nothing to disclose. Dr. Aguirrebengoa has nothing to disclose. Dr. Vera-Mendez reports personal fees from BMS, ABBVIE, MSD and Janssen-Cilag, outside the submitted work. Dr. Ferrer is an employee of Gilead Sciences SL and may own stocks and/or stock options in the company. Dr. Blanco reports grants and personal fees from Gilead Sciences, during the conduct of the study; grants and personal fees from Abbott Laboratories and Bristol-Myers Squibb; personal fees from Janssen, Merck and ViiV Healthcare, and grants and personal fees from Gilead Sciences, outside the submitted work.
Ethics Approval
This study was approved by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Acknowledgements
This study would not have been possible without the collaboration of the TRIP study group and of all the patients, physicians and nurses who have taken part in the project.
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents and Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents – Last updated February 12, 2013. Section: HIV and the older patient, page I31. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed March 03, 2014..
- Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201.
- Gallant JE, Winston JA, Dejesus E, et al. The 3-year renal safety of a tenofovir disoproxil fumarate vs. a thymidine analogue-containing regimen in antiretroviral-naive patients. AIDS. 2008;22:2155–2163.
- Campbell LJ, Ibrahim F, Fisher M, Holt SG, Hendry BM, Post FA. Spectrum of chronic kidney disease in HIV-infected patients. HIV Med. 2009;10:329–336.
- Choi AI, Shlipak MG, Hunt PW, Martin JN, Deeks SG. HIV-infected persons continue to lose kidney function despite successful antiretroviral therapy. AIDS. 2009;23:2143–2149.
- O'Donnell EP, Scarsi KK, Darin KM, Gerzenshtein L, Postelnick MJ, Palella FJ Jr. Low incidence of renal impairment observed in tenofovir-treated patients. J Antimicrob Chemother. 2011;66:1120–1126.
- Mocroft A, Kirk O, Gatell J, et al. Chronic renal failure among HIV-1-infected patients. AIDS. 2007;21:1119–1127.
- Izzedine H, Isnard-Bagnis C, Hulot JS, et al. Renal safety of tenofovir in HIV treatment-experienced patients. AIDS. 2004;18:1074–1076.
- Deti EK, Thiebaut R, Bonnet F, et al. Prevalence and factors associated with renal impairment in HIV-infected patients, ANRS C03 Aquitaine Cohort, France. HIV Med. 2010;11:308–317.
- Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126.
- Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–1139.
- Blanco JR, Caro-Murillo AM, Castano MA, et al. Safety, efficacy, and persistence of emtricitabine/tenofovir versus other nucleoside analogues in naive subjects aged 50 years or older in Spain: the TRIP study. HIV Clin Trials. 2013;14:204–215.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Gupta SK, Eustace JA, Winston JA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40:1559–1585.
- Winston J, Deray G, Hawkins T, Szczech L, Wyatt C, Young B. Kidney disease in patients with HIV infection and AIDS. Clin Infect Dis. 2008;47:1449–1457.
- Fux CA, Simcock M, Wolbers M, et al. Tenofovir use is associated with a reduction in calculated glomerular filtration rates in the Swiss HIV Cohort Study. Antivir Ther. 2007;12:1165–1173.
- Crum-Cianflone N, Ganesan A, Teneza-Mora N, et al. Prevalence and factors associated with renal dysfunction among HIV-infected patients. AIDS Patient Care STDS. 2010;24:353–360.
- Kinai E, Hanabusa H. Progressive renal tubular dysfunction associated with long-term use of tenofovir DF. AIDS Res Hum Retroviruses. 2009;25:387–394.
- Young J, Schafer J, Fux CA, et al. Renal function in patients with HIV starting therapy with tenofovir and either efavirenz, lopinavir or atazanavir. AIDS. 2012;26:567–575.
- Gallant JE, Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. 2009;23:1971–1975.
- Goicoechea M, Liu S, Best B, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008;197:102–108.
- Young B, Buchacz K, Moorman A, Wood KC, Brooks JT. Renal function in patients with preexisting renal disease receiving tenofovir-containing highly active antiretroviral therapy in the HIV outpatient study. AIDS Patient Care STDS. 2009;23:589–592.
- Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2006;43:278–283.
- Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT Study. J Acquir Immune Defic Syndr. 2010;55:49–57.
- Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis. 2006;42:283–290.
- Lubomirov R, Colombo S, di IJ, et al. Association of pharmacogenetic markers with premature discontinuation of first-line anti-HIV therapy: an observational cohort study. J Infect Dis. 2011;203:246–257.
- Nishijima T, Gatanaga H, Komatsu H, et al. Renal function declines more in tenofovir-than abacavir-based antiretroviral therapy in low-body weight treatment-naive patients with HIV infection. PLoS One. 2012;7:e29977.
Appendix: The TRIP study group
José Ramón Blanco, José Antonio Oteo, Valvanera Ibarra (Hospital San Pedro CIBIR, Logroño); Enric Pedrol, Sheila Ruiz, María Tasias, Antonio Delegido (Hospital Sant Pau i Santa Tecla, Tarragona); Manuel Castaño, María Isabel Mayorga (Hospital Carlos Haya, Málaga); Julián Olalla, Alfonso del Arco, Javier de la Torre, José Luis Prada (Hospital Costa del Sol, Marbella); Pere Domingo, Jéssica Muñoz (Hospital Sant Pau, Barcelona); Piedad Arazo, Ascensión Pascual, Desiré Gil (Hospital Universitario Miguel Servet, Zaragoza); Juan Luis Gómez Sirvent, Ma Mar Alonso, Remedios Alemán, Dácil García, Ricardo Pelazas, Patricia Rodríguez (Hospital Universitario de Canarias, Santa Cruz de Tenerife); Melchor Riera (Hospital Son Espases, Palma de Mallorca); Federico Pulido, Asunción Hernando, María de Lagarde (Hospital Universitario 12 de Octubre, Madrid); Francisco Vera (Hospital Santa Lucía, Cartagena); Antonio Vergara, Alberto Romero, Patricia Jiménez, Efraín Cruz, José Javier Borrallo (Hospital Universitario de Puerto Real, Puerto Real); Koldo Aguirrebengoa, Mónica de Miguel, Rosa Martínez, Yolanda Rodríguez (Hospital de Cruces, Bilbao); Joaquín Portilla, Vicente Boix, Esperanza Merino, Sergio Reus, Silvia García (Hospital General de Alicante, Alicante); Jesús Rodríguez Baño, Carmen Machado, Virginia Palomo (Hospital Virgen de la Macarena, Sevilla); Jorge Vergas (Hospital Clínico San Carlos, Madrid); José Hernández Quero (Hospital Universitario San Cecilio, Granada); Bonaventura Clotet, Inés Fernández (Fundación Lluita contra la Sida, Badalona).