Abstract
Background:
Despite combination antiretroviral therapy (cART), 20% of HIV-infected patients are unable to achieve adequate immunologic recovery, in which immune activation plays a crucial role. We hypothesize that extract of Tripterygium wilfordii Hook F (TwHF), a Chinese medication used to treat autoimmune diseases, has immunomodulatory effects that may help CD4 cell recovery.
Methods:
Eighteen cART-treated HIV-infected patients virally suppressed for over 12 months with suboptimal CD4 cell recovery were enrolled. TwHF extract was administered at a dosage of 10 mg three times daily for 12 months. T-cell subsets and activation markers were evaluated at baseline and during follow-up. The trial was registered at Clinicaltrials.gov (NCT02002286).
Results:
TwHF extract was associated with a mean increase in CD4 cell count of 88 cells/μl (95% confidential interval [CI], 72–105 cells/μl) after one year of treatment. A significant increase in the mean rate of CD4 cell recovery (26 before vs 75 cells/μl/year after TwHF use, P < 0.001) was observed. Analysis of 13 patients with activation profiles suggested that TwHF extract was associated with a decrease in T-cell immune activation which was temporally correlated with CD4 cell recovery. No discontinuation of TwHF extract was reported.
Conclusion:
Use of TwHF extract in HIV-infected patients was associated with a reduction in T-cell activation and improved CD4 recovery with an excellent safety profile.
Introduction
Although combination antiretroviral therapy (cART) has substantially improved patient outcomes by suppressing HIV-1 replication leading to sustained CD4 cell recovery for most HIV-infected patients,Citation1 approximately 20% of patients do not achieve adequate CD4 cell reconstitution despite prolonged virologic suppression. Typically, the CD4 cell recovery of these patients, called immunologic non-responders (INRs),Citation2 plateaus beyond 10 years. Factors including advanced age (>45 years), low nadir CD4 cell count, higher baseline viral load, reduced thymic output, a history of AIDS-defining events, and especially increased immune activation are the main driving force behind poor CD4 cell recovery.Citation2–Citation7 An inadequate immunologic response is associated with a higher risk of opportunistic infections,Citation8,Citation9 cardiovascular disease,Citation10 renal disease,Citation11 malignancy,Citation12 neurological complications,Citation13 as well as higher mortality.Citation9,Citation12 Specific medications including hydroxychloroquine (HCQ), chloroquine and celecoxib dampen immune activation in HIV infection.Citation14–Citation17 In virally suppressed INRs treated with cART and HCQ, however, only a trend toward CD4 cell count recovery was observed,Citation14 while studies using chloroquine and celecoxib in HIV-patients not on cART failed to show a significant increase in CD4 cell count.Citation15–Citation17 To date, no immunomodulants have been shown to promote CD4 cell recovery significantly.Citation18 Finding an effective therapy that decreases the level of immune activation in HIV infection and enhances immune recovery addresses an unmet need.
The extract of Tripterygium wilfordii Hook F (TwHF), a traditional Chinese medication, has been utilized as an anti-inflammatory therapy to treat different autoimmune diseases including rheumatoid arthritis (RA)Citation19 and Crohn's disease.Citation20 A recent multicenter, open-label, randomized controlled trial in China comparing TwHF and methotrexate showed that TwHF was not inferior to methotrexate monotherapy in controlling acute RA.Citation21 Multiple in vitro and in vivo studies have demonstrated that triptolide, the major bioactive component extracted from TwHFCitation22 (chemical structure shown in Fig. S1 see supplementary material online for this article www.maneyonline.com/doi/suppl/10.1179/1528433614Z.0000000005), can reduce lipopolysaccharide-induced inflammation,Citation23–Citation26 a crucial factor for immune activation in HIV-1 infection.Citation24,Citation27,Citation28 In addition, triptolide inhibits proinflammatory markers like COX-2 and iNOSCitation26 and suppresses their proinflammatory downstream signals.Citation25
In this pilot study, we evaluated the efficacy of TwHF extract on immune activation and immune recovery.
Methods
Patients
Eighteen HIV-infected patients at Peking Union Medical College Hospital Clinic receiving cART for at least two years with plasma HIV-1 viral load < 40 copies/ml and suboptimal CD4 cell recovery were enrolled after informed consent was obtained. Patients were recruited from August 1, 2011 to August 1, 2012 and were followed until August 1, 2013 ().
Definitions
Immune non-responders were defined as patients with CD4 cell count < 200 cells/μl or < 20% increase in CD4 cell count compared with baseline after at least 12 months; inadequate responders (InaR) were defined as patients with CD4 count between 200 and 300 cells/μl after at least12 months of viral suppression.Citation2,Citation3
Study design
TwHF extract was administered orally at a dosage of 10 mg three times daily for 12 months. Patients also continued on their usual cART regimen. The primary endpoints were changes in CD4 cell count and in T-cell subsets. The secondary endpoints were immune activation levels and the safety profile. T-cell subsets and activation markers were evaluated at baseline and at weeks 12, 24, and 48.
HIV-1 viral load and T-cell subsets
Measurement of plasma HIV-1 viral load has been described.Citation29 In brief, viral load was measured with COBAS Ampliprep/TaqMan48 real-time RT-PCR Test (Roche Diagnostics) with HIV-1 viral detection range of 40 to 10 000 000 copies/ml. T-cell subsets and immune activation were measured using six-color FACSCanto flow cytometer (BD Biosciences) to detect, CD4(+) T cells (CD4(+)CD3(+)), CD8+T cells (CD8(+)CD3(+)), naive CD4+T cells (CD4(+)CD45RA(+)CD62L(+)), memory CD4+T cells (CD4(+)CD45RA( − )), and activated T cells (CD38(+)HLA-DR(+)), as described previously.Citation3
Statistical analysis
Noncategorical baseline variables were analyzed using unpaired t test (data with normal distribution) or Mann–Whitney U test (data with non-normal distribution). Categorical variables were analyzed with chi square test or Fisher's exact test. CD4 cell recovery was evaluated using one-way ANOVA with repeated measures. Generalized estimating equations (GEE) were utilized to evaluate slopes of CD4 cell recovery. We also used linear regression to calculate CD4 cell recovery rate in each patient and used paired t test to compare differences in CD4 cell recovery rate before and after TwHF use, which is a method utilized in previous studies.Citation30,Citation31 Wilcoxon paired test was utilized to evaluate changes in immune activation.Citation15 Spearman's rank-correlation was used to evaluate the correlation between immune activation and CD4 cell recovery. Normality was tested by using the Shapiro-Wilk test. For all tests, P < 0.05 was considered to be statistically significant. SPSS 20.0 statistical package was used for all analyses.
Role of the funding source
The funders had no role in study design, data collection, data analyses, preparation of the manuscript, or decision to publish.
Ethics statement
Informed consent was obtained from every participant, and the study was approved by the Institutional Review Board of Peking Union Medical College Hospital. It was in compliance with local regulations and the Declaration of Helsinki.
Results
Baseline characteristics
Baseline characteristics are summarized in . Demographic and clinical characteristics were similar between INR and InaR except for baseline and nadir CD4 count as well as duration of cART use and viral suppression. Most patients had been on cART for over two years and virally suppressed for more than one-and-a-half years.
Table 1. Population characteristics
Total and memory CD4 cells increased during TwHF treatment
Immunologic response before and after TwHF extract administration is summarized in . After one year of TwHF extract use, CD4 cell count increased by a mean of 88 cells/μl (95% confidential interval [CI] 72–105 cells/μl). In both INR and InaR, a significant increase in CD4 cell count was observed ( and Supplementary in Supplementary Material). Importantly, a rise in memory CD4 cells accounted for this increase rather than naive cells ( and Supplementary see supplementary material online for this article www.maneyonline.com/doi/suppl/10.1179/1528433614Z.0000000005). CD4 cell recovery for each patient is shown in .
Figure 2. Association between TwHF extract and CD4 cell recovery. (A–C) an increase in total and memory CD4 cell count was observed during TwHF use. In B and C, data from 14 patients were available for T-cell subgroup analysis (n = 7 in INR group and n = 7 in InaR group). In Fig. 2A–C, gray asterisks indicate a significant difference between T-cell subsets before TwHF use and at TwHF initiation; black asterisks indicate a significant difference between T-cell subsets after TwHF use and at TwHF initiation. Dashed lines indicate TwHF initiation. (D, E) increase in total and memory CD4 T cells calculated by generalized estimating equation (GEE). Rates of CD4 cell recovery and 95% confidential intervals (CI) before and after TwHF extract use are labeled in these figures. (F–H) increase rates of CD4 cell and subgroups before and after TwHF extract use. The rate of increase in each patient was derived from linear regression, and the comparison was made using paired t-test, with changes in mean (95% CI) rates of increase labeled in these figures. *, 0.01 < P < 0.05; * *, 0.001 < P ≤ 0.01; * * *, P ≤ 0.001.
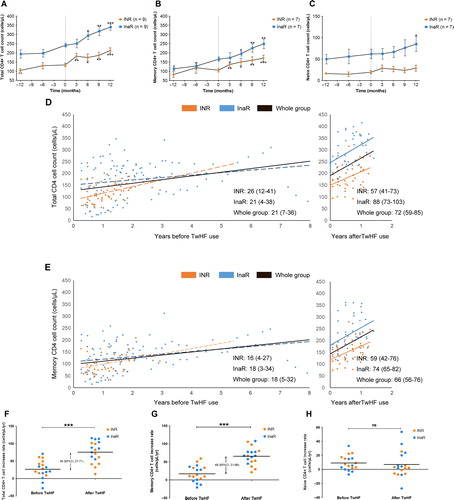
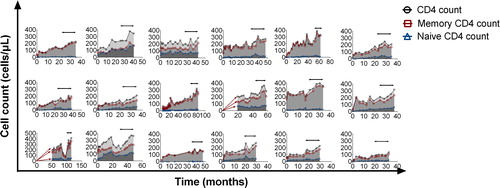
Figure 3. CD4 cell recovery for each patient. Horizontal lines with arrows indicate TwHF extract use. cART was administered at the beginning of each graph.
We calculated the slope of CD4 cell increase before and after TwHF extract initiation. The rate of increase before TwHF extract use was calculated by linear regression of CD4 cell recovery from three months after cART (second phase of CD4 cell increaseCitation3) to initiation of TwHF extract. In comparison with the rates of CD4 cell count increase in the slow increase phase, rates of increase were significantly higher after TwHF extract administration (); in addition, memory CD4 cell recovery accounted for total CD4 cell recovery (). This was further confirmed by comparing slopes of CD4 total T-cell and memory T-cell increase (), in which significant increases in mean CD4 cell recovery rates (26 before vs 75 cells/μl/year after TwHF extract use, P < 0.001) and mean memory CD4 cell recovery rates (17 before vs 65 cells/μl/year after TwHF extract use, P < 0.001) were observed after TwHF extract administration. We also observed changes in CD8 cells and the CD4/CD8 ratio (see Fig. S2 in supplementary material online for this article www.maneyonline.com/doi/suppl/10.1179/1528433614Z.0000000005).
Association between TwHF use and decrease in immune activation
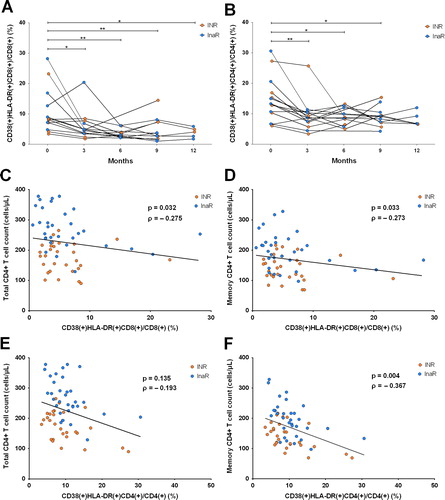
We next explored the potential mechanisms by which TwHF extract promotes CD4 cell recovery. The median percentage of CD38(+)HLA-DR(+)CD8(+) T cells decreased significantly during the 12-month treatment period () Importantly, the decrease in immune activation was most prominent in the first three months (), which preceded the recovery of CD4 cells and memory CD4 cells in the InaR and INR groups (). Similarly, the percentage of CD38(+)HLA-DR(+)CD4(+) cells also decreased ().
Figure 4. Association between TwHF extract use and immune activation in patients with poor immune response. (A, B) CD8 and CD4 cell activation profiles during TwHF extract treatment (n = 13 in month 0 and 3, n = 10 in month 6, n = 11 in month 9, n = 5 in month 12). P values were derived by using Wilcoxon paired test. Median level of immune activation is also labeled in figures. (C–F) correlation between CD38(+)HLA-DR(+) double positive CD8 or CD4 T-cell percentage and total or memory CD4 cell count. *, 0.01 < P < 0.05; * *, 0.001 < P ≤ 0.01; * * *, P ≤ 0.001.
Association between immune activation and CD4 cell recovery
We last assessed the association between CD8(+) T-cell immune activation and CD4 cell recovery after TwHF extract treatment. Total CD4 cell count was negatively correlated with CD38(+)HLA-DR(+)/CD8(+) percentage () Similar results were also observed in memory CD4 cells (). Interestingly, CD4 cell activation was negatively correlated with CD4 T memory cell counts, but not with total CD4 cell counts ().
Safety profile
Throughout the 12-month treatment period, TwHF extract was well tolerated. No discontinuation of TwHF extract was reported. Two patients developed hypercholesterolemia and one patient developed transient thrombocytopenia (Grade II or higher per the Division of AIDS grading of severity of adult and pediatric adverse events, http://www.hptn.org/web%20documents/hptn046/ssp/appendices/appendixe-toxicitytables_daids_ae_gradingtable_finaldec2004.pdf) after TwHF administration. No other TwHF-associated adverse effects including hematologic complications, infection, hepatic impairment, gastrointestinal events greater than Grade II were reported. Participants remained virally suppressed throughout the trial, although occasional isolated blips (defined as a transiently detectable low viral load, typically less than 400 copies/ml, at no more than one visit) were observed.
Discussion
This is the first study to demonstrate the association of TwHF extract, a well-tolerated traditional Chinese medication, with immune recovery and decreased immune activation.
We showed an association between low dose TwHF extract and reduced T-cell activation in the setting of viral suppression on cART. In addition, this immune modulatory effect was temporally correlated to immune recovery: decreased CD8 cell activation correlated to total CD4 cell and memory CD4 cell recovery, while decreased CD4 cell activation correlated in particular to memory CD4 cell recovery. Taken together, these results are consistent with previous findings that HIV infection and immunologic failure are related to preferential depletion of memory CD4 cells, which is correlated with immune activation.Citation32–Citation34 Thus, as immune activation decreases with TwHF extract use, memory CD4 cells are able to recover. Notably, the CD4/CD8 ratio did not normalize with TwHF use, reflecting the expansion of effector CD8 cells which are associated with aging and accelerated disease progression. Interventions aimed at fully restoring this ratio should be investigated.Citation35
Previously, several approaches targeting immune activation have been attempted.Citation18,Citation36 HCQ has been added to cART in the setting of viral suppression.Citation14 It had significant immunomodulatory effects and reduced inflammation and immune activation; however, only a trend toward CD4 cell count recovery was observed.Citation14 This may be partially explained by differences in immune modulation between TwHF and HCQ, as HCQ did not show a significant decrease in CD38 expression,Citation14 while in our study, TwHF did. In addition, whether the multiple immune activation and proinflammatory markers utilized in the HCQ study are predictive of clinical outcomes needs further assessment.
Notably, the observed immunomodulatory effect is useful only when virologic suppression has been achieved, given that its target is ‘residual’ immune activationCitation3 due to low level plasma viremiaCitation37 and microbial translocation.Citation27 In another study using HCQ in cART-naive patients, HCQ failed to decrease CD8 cell activation and was associated with poorer clinical outcomes compared with placebo.Citation17 Other small-scale studies with chloroquine,Citation15 celecoxib,Citation16 and leflunomideCitation38 showed suppression of immune activation in the absence of cART and viral suppression but failure to boost CD4 recovery. These results suggest that immunomodulants exert optimal effects only after viral suppression.
Low dose TwHF extract is sufficient to suppress immune activation without causing significant adverse effects greater than Grade II. Since TwHF extract has been widely used in China for the treatment of RA and other autoimmune diseases including Crohn's disease and certain types of nephrotic syndrome at a dosage of 20 mg three times daily,Citation21 its safety profile is well-established. However, because it has never been used in HIV-infected patients for this purpose, we reduced the dosage in an attempt to minimize adverse effects including hematologic complications or infections.
While this study is the first to identify a medication associated with a decrease in immune activation, and most importantly, CD4 cell recovery in HIV-infected patients, there are some limitations to our study. First, this is a pilot study with a limited sample size and, thus, limited power. The study was also not a randomized, placebo-controlled trial. As such, we were only able to demonstrate an association between TwHF and CD4 cell recovery and cannot draw any conclusions regarding causality. Based on our results, however, a randomized, placebo-controlled, multicenter study is warranted, in addition to further investigations regarding the mechanism of TwHF.
In conclusion, this pilot study establishes TwHF extract as an effective immunomodulant, and provides new strategies for targeting immune activation, inadequate immune response, and, potentially, for associated comorbidities. Further randomized placebo-controlled studies are warranted to evaluate the immunomodulatory effects of TwHF extract on CD4 cell recovery.
Acknowledgements
We thank Felicia C. Chow from University of California, San Francisco for her help in revising the manuscript. We are grateful for all the healthcare providers and all the patients who have participated in these studies.
Disclaimer Statements
Contributors
T. Li and J.-P. Routy managed and designed the research, coordinated the patient cohort, and provided critical review of the manuscript. J. Xie, Y. Han, Z. Qiu, M. Sun and X. Zhang performed primary study assays. Y.J. Li, J. Xie and T. Li analyzed data and wrote the manuscript. Y.L. Li and X. Song participated in patient management, implemented the clinical protocol and data collection. W. Lv, F. Wang and H. Jiang participated in data analyses.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81071372 to T. Li) and National Key Technologies R&D Program for the 12th Five-year Plan (grant no. 2012ZX10001003-001 to T. Li). J. Xie was supported by the grant of Chinese Ministry of Human Resources and Social Security and Peking Union Medical College Hospital Research Fund for Young Investigators. This study received partial support from Canadian Institute of Health, Clinical HIV trials network, to J.-P. Routy. In addition, J.-P. Routy holds the McGill University Louis Lowenstein Chair of Hematology & Oncology, Montreal, QC, Canada.
Conflicts of Interests
The authors declare no conflicts of interest.
Ethics Approval
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital. It was in compliance with local regulations and the Declaration of Helsinki.
References
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116.
- Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol. 2012;2012:670957.
- Li T, Wu N, Dai Y, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis. 2011;53:944–951.
- Massanella M, Negredo E, Perez-Alvarez N, et al. CD4 T-cell hyperactivation and susceptibility to cell death determine poor CD4 T-cell recovery during suppressive HAART. AIDS. 2010;24:959–968.
- Nakanjako D, Ssewanyana I, Mayanja-Kizza H, et al. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis. 2011;11:43.
- Gascon RL, Narvaez AB, Zhang R, et al. Increased HLA-DR expression on peripheral blood monocytes in subsets of subjects with primary HIV infection is associated with elevated CD4 T-cell apoptosis and CD4 T-cell depletion. J Acquir Immune Defic Syndr. 2002;30:146–153.
- Bosch RJ, Wang R, Vaida F, Lederman MM, Albrecht MA. Changes in the slope of the CD4 cell count increase after initiation of potent antiretroviral treatment. J Acquir Immune Defic Syndr. 2006;43:433–435.
- Mocroft A, Furrer HJ, Miro JM, et al. The incidence of AIDS-defining illnesses at a current CD4 count ≥ 200 cells/μL in the post-combination antiretroviral therapy era. Clin Infect Dis. 2013;57:1038–1047.
- Lundgren JD, Babiker A, El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–1155.
- Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624.
- Maggi P, Bartolozzi D, Bonfanti P, et al. Renal complications in HIV disease: between present and future. AIDS Rev. 2012;14:37–53.
- Monforte A, Abrams D, Pradier C, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–2153.
- Mothobi NZ, Brew BJ. Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis. 2012;25:4–9.
- Piconi S, Parisotto S, Rizzardini G, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118:3263–3272.
- Murray SM, Down CM, Boulware DR, et al. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol. 2010;84:12082–12086.
- Pettersen FO, Torheim EA, Dahm AE, et al. An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downregulated immune activation and improved T cell-dependent vaccine responses. J Virol. 2011;85:6557–6566.
- Paton NI, Goodall RL, Dunn DT, et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308:353–361.
- Rajasuriar R, Khoury G, Kamarulzaman A, French MA, Cameron PU, Lewin SR. Persistent immune activation in chronic HIV infection: do any interventions work? AIDS. 2013;27(8):1199.
- Goldbach-Mansky R, Wilson M, Fleischmann R, et al. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Ann Intern Med. 2009;151, 229–240, W249–251.
- Ren J, Tao Q, Wang X, Wang Z, Li J. Efficacy of tb2 in active Crohn's disease: a prospective study report. Dig Dis Sci. 2007;52:1790–1797.
- Lv QW, Zhang W, Shi Q, et al. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann Rheum Dis. 2014;pii, annrheumdis-2013-204807.
- Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol. 2012;74:424–436.
- Lu Y, Bao X, Sun T, Xu J, Zheng W, Shen P. Triptolide attenuate the oxidative stress induced by LPS/D-GalN in mice. J Cell Biochem. 2012;113:1022–1033.
- Matta R, Wang X, Ge H, Ray W, Nelin LD, Liu Y. Triptolide induces anti-inflammatory cellular responses. Am J Transl Res. 2009;1:267–282.
- Premkumar V, Dey M, Dorn R, Raskin I. MyD88-dependent and independent pathways of Toll-Like Receptors are engaged in biological activity of Triptolide in ligand-stimulated macrophages. BMC Chem Biol. 2010;10:3.
- Ma J, Dey M, Yang H, et al. Anti-inflammatory and immunosuppressive compounds from Tripterygium wilfordii. Phytochemistry. 2007;68:1172–1178.
- Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371.
- Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133.
- Wang H, Li Y, Zhang C, et al. Immunological and virological responses to cART in HIV/HBV co-infected patients from a multicenter cohort. AIDS. 2012;26:1755–1763.
- Benito JM, Lopez M, Lozano S, et al. CD4+T cell recovery beyond the first year of complete suppression of viral replication during highly active antiretroviral therapy is not influenced by CD8+T cell activation. J Infect Dis. 2005;192:2142–2146.
- Benito JM, Lopez M, Lozano S, et al. Differential upregulation of CD38 on different T-cell subsets may influence the ability to reconstitute CD4+T cells under successful highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;38:373–381.
- Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226.
- Kolber MA. CD38+CD8+T-cells negatively correlate with CD4 central memory cells in virally suppressed HIV-1-infected individuals. AIDS. 2008;22:1937–1941.
- Zhou Y, Shen L, Yang HC, Siliciano RF. Preferential cytolysis of peripheral memory CD4+T cells by in vitro X4-tropic human immunodeficiency virus type 1 infection before the completion of reverse transcription. J Virol. 2008;82:9154–9163.
- Emu B, Moretto WJ, Hoh R, et al. Composition and function of T cell subpopulations are slow to change despite effective antiretroviral treatment of HIV disease. PLoS One. 2014;9:e85613.
- Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10:655–666.
- Taiwo B, Hunt PW, Gandhi RT, et al. CD8+T-Cell Activation in HIV-1-infected patients experiencing transient low-level viremia during antiretroviral therapy. J Acquir Immune Defic Syndr. 2013;63:101–104.
- Read SW, DeGrezia M, Ciccone EJ, et al. The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants. PLoS One. 2010;5:e11937.