Abstract
Objectives:
The aim of this study was to determine the effectiveness and safety of rilpivirine in treatment-naive adults infected with HIV-1.
Methods:
We ran duplicate searches of multiple databases and searchable websites of major HIV conferences (up to October 2013) to identify randomized controlled trials reporting the effectiveness and safety of rilpivirine in treatment-naive adults infected with HIV-1. Reference lists from retrieved articles were also reviewed. Data were extracted independently in duplicate using predefined data fields. All analyses used random-effects models to calculate the summary treatment effect estimates.
Results:
Four randomized controlled trials with a total of 2522 patients were included in the inclusion criteria. The primary efficacy endpoint was the proportion of patients with confirmed HIV-1 RNA levels of < 50 copies/ml (viral load) at 48 weeks. Rilpivirine demonstrated non-inferior antiviral efficacy in viral load comparable with efavirenz at 48 weeks [relative risk (RR) = 1.03, 95% confidence interval (CI): 0.99–1.07]. The mean changes from baseline in CD4 count were similar in both rilpivirine and efavirenz (RR = 1.05, 95% CI: 0.85–1.24). Rilpivirine showed higher and significant difference in virological failure rates comparing with the efavirenz group (RR = 1.70, 95% CI: 1.21–2.38). The incidences of the most commonly reported adverse events related to study medication, including rash, and neurological events, were lower with rilpivirine than with efavirenz (RR = 0.11, 95% CI: 0.03–0.33; RR = 0.52, 95% CI: 0.45–0.60, respectively).
Conclusions:
Current evidence suggests a range of favorable effects and a generally favorable safety profile of rilpivirine in treatment-naive adults infected with HIV-1 at week 48.
Introduction
Efavirenz (EFV) is a first-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) that is recommended by several treatment guidelines in combination with tenofovir-disoproxilfumarate and emtricitabine.Citation1–Citation3 However, EFV is associated with several adverse events, including neurological and psychiatric adverse events, rash, and teratogenicity, which can lead to discontinuation of therapy. Furthermore, EFV is contraindicated for pregnant women because of concerns over potential teratogenicity.Citation4,Citation5 Therefore, new, potent NNRTIs with activity against NNRTI-resistant viruses and better tolerability are needed.
Rilpivirine (RPV) is the next-generation diarylpyrimidine NNRTI that is highly effective in treating wild-type and drug-resistant HIV-1 infections in clinical trials at relatively low doses (about 25–75 mg/day).Citation6–Citation9 RPV has recently been approved in several countries worldwide, including the USA, Canada, and Europe, in combination with other ARVs for the treatment of HIV-1-infected ARV-naive adult patients.Citation10,Citation11
Although there have been trials assessing the effectiveness and safety of RPV,Citation12–Citation19 to our knowledge, no quantitative, systematic review of this literature has been undertaken. Below we review and synthesize the published evidence from randomized trials evaluating the association of the effectiveness and safety of RPV in treatment-naive adults infected with HIV-1. We decided to follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines in the report of this meta-analysis.Citation20
Methods
Search strategy
We performed a systematic literature review up to October 2013, with the help of Medline, EMBASE, CINAHL, the Cochrane Controlled Trial Register database, CBMdisc, and Chinese Medical Current Contents using the search terms: effectiveness or evaluation or impact or result; HIV or AIDS; rilpivirine; a non-nucleoside reverse transcriptase inhibitor or NNRTI; efavirenz; and randomized controlled trials (RCTs).
The search strategy was developed without any language restriction. We evaluated potentially relevant publications by examining their titles and abstracts and then procured the most relevant publications for a closer examination. The search was supplemented by manually reviewing the reference list of retrieved articles and relevant reviews and contacting content experts for additional published or unpublished trials. The search and evaluation were conducted from May to October 2013.
Study selection
One of us (Sun) did a preliminary search scanning all titles for eligibility according to predefined inclusion criteria. Results from database searches combined and duplicates were removed. Each title and abstract was reviewed to determine eligibility. After obtaining full abstracts for potentially eligible studies, two reviewers (Li and Zhang) worked independently to assess eligibility. A paper was rejected if it was obvious from title/abstract review that it was ineligible. When it was less clear, the full paper was reviewed. Two of the authors (Xu and Sun) performed study selection independently, with disagreements resolved through discussion and by the opinion of a third reviewer (Lu), if necessary.
Inclusion and exclusion criteria
Studies were included in this meta-analysis if they met the following criteria: (1) had a prospective cohort study design; (2) patients aged 18 years or older, who had not been previously treated with antiretroviral drugs, a plasma viral load at screening of 5000 copies/ml or greater, and viral sensitivity to tenofovir–disoproxil–fumarate and emtricitabine.Citation13–Citation15
Further exclusion criteria included infection with HIV-2, documented evidence of at least one NNRTI resistance-associated mutation from a list of 39(A98G, L100I, K101E/P/Q, P225H, fig227C, K103H/N/S/T, V106A/M, V108I, E138A/G/K/Q/R, V179D/E, Y181C/I/V, P236L, K238N/T,Y188C/H/L, G190A/C/E/Q/S/T, M230I/L, and Y318F),Citation21 any active clinically significant disease (e.g. pancreatitis, cardiac dysfunction, active and significant psychiatric disorder, adrenal insufficiency, hepatic impairment), renal impairment (estimated glomerular filtration rate based on creatinine < 50 ml per min) and, for women, pregnancy or breastfeeding.Citation13–Citation15
Data extraction
Two investigators (Li and Zhang) extracted all the data independently in duplicate by means of a standardized protocol and data collection form. Disagreements were resolved by discussion with a third investigator (Lu).
Data abstractors collected information about first author's name, year of publication, the study setting, study populations, sample size, total number of cases and controls, trial length, and study design. Our primary efficacy endpoint was the proportion of patients with confirmed HIV-1 RNA levels of < 50 copies/ml at 48 weeks, analyzed by the time to loss of virological response algorithm; secondary outcomes were CD4 cell counts, virological failure, rash events, and neurological events. Unsystematic observations (case series or case reports) were excluded from all analyses.
We applied the GRADE system and Jadad scale to assess the randomization, blinding and reports of dropouts and withdrawals for the trials.Citation22,Citation23 Each trial was independently scored by two investigators (Li and Sun) and any areas of disagreement arbitered by a third investigator (Lu). In the final, the weighted score was 4.0 for Jadad score, and thus, the qualities of the included studies were “high quality” (Jadad et al. recommended that a score of 3 or more for the trial to be designated “high quality” and included in the meta analysis, a score of less than 3 meant that the trial was designated “low quality”).
Data analysis
Li and Zhang conducted all statistical analyses; relative risk (RR) and their 95% confidence interval (CI) were used to assess the effectiveness and safety of RPV in treatment-naive adults infected with HIV-1. We pooled data using the DerSimonian–Laird random effects method, which recognizes and anchors studies as a sample of all potential studies, and incorporates an additional between-study component to the estimate of variability.Citation24 Therefore, the random-effects model was applied in our meta-analysis to generate a more conservative estimate of the proportion.
We performed a test-based Q statistic test and calculated the I2 statistic (I2 confidence intervals) for each analysis to assess the between-study heterogeneity.Citation25 P < 0.1 indicates significant heterogeneity. Publication bias was examined with Egger's and Begg's tests. The bias (such as publication and location bias) was investigated by funnel plot. An asymmetric plot suggested possible publication bias. It was assessed quantitatively by a linear regression test that Egger et al. defined.Citation26 A regression line which passes through the origin of the plot (within error limits) indicates symmetry and hence, the absence of bias. The significance of the intercept was determined by the t-test as suggested by Egger. Statistical significance was taken as P < 0.05. All analyses were carried out using STAT software, version 11.0 (STATA Corp., College Station, TX, USA), and all tests were two-sided.
Results
Search result
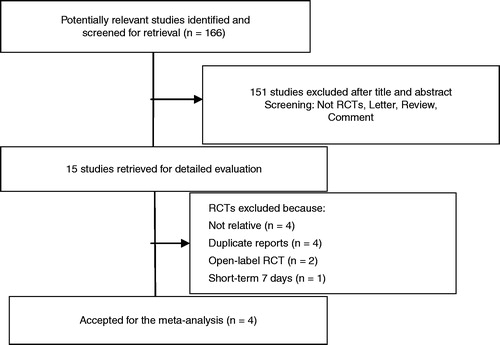
We initially identified 166 unique citations from the PubMed database, EMBASE, Cochrane Library, CBM, and CNKI databases, using different combinations of key terms. All languages were included. Assessment of title and/or abstract excluded a further 151 articles that did not meet inclusion criteria. Fifteen trials were identified for potential inclusion in the study, all randomized trials. Four trials were not relevant to our analysis,Citation17,Citation18,Citation27,Citation28 four trials overlapped with included trials,Citation29–Citation32 two studies open-label randomized trials,Citation16 one was of short-term randomized trial.Citation12 Four trials remained for this study and are described in .Citation13–Citation15,Citation19 shows the flow diagram of study selection for analysis.
Table 1. Baseline characteristics of the patients in four RCTs
Quality assessment of included studies
After reviewing every trial, we identified four original trials.Citation13–Citation15,Citation19 The information about each trial is shown in . Demographics and baseline characteristics were balanced between the RPV and EFV groups. Four randomized controlled trials with a total of 2522 patients were included in this review. All of the trials pointed out the randomization. Double-blind trials were described. Follow-up was used from 48 to 96 weeks.
Meta-analysis
Virological response: viral load
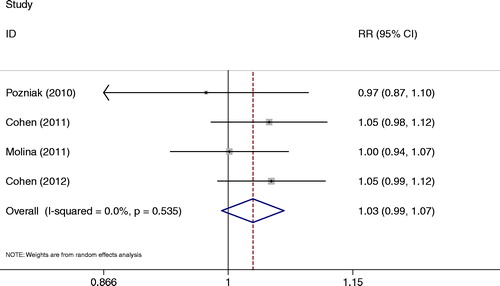
Each studyCitation13–Citation15,Citation19 reported the viral load at week 48. There was no significant difference in the viral load by comparing the RPV group with the EFV group (RR = 1.03, 95% CI: 0.99–1.07) (). The heterogeneity between the studies was not significant (P = 0.535, I2 = 0.0%).
CD4 T lymphocyte
All studiesCitation13–Citation15,Citation19 showed CD4 cell counts at week 48. Increases in CD4 cell counts were much the same between the RPV group and the EFV group (RR = 1.04, 95% CI: 0.85–1.24). There was a significant heterogeneity between the studies (P = 0.03, I2 = 70.6%).
Virological failure
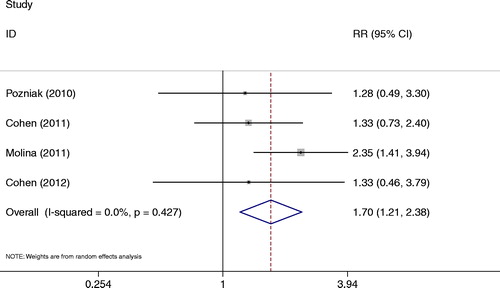
Each studyCitation13–Citation15,Citation19 reported virological failure at week 48. There was higher and significant difference in this index in comparing the RPV group with the EFV group (RR = 1.70, 95% CI: 1.21–2.38) (); no heterogeneity was observed between studies (P = 0.427, I2 = 0.00%).
Rash events
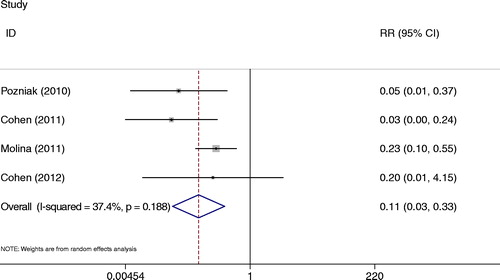
All studiesCitation13–Citation15,Citation19 presented rash events at week 48. The incidence of any rash possibly related to treatment (any grade) was lower for RPV than for EFV (RR = 0.11, 95% CI: 0.03–0.33) (). There was little heterogeneity among four randomized trials with this outcome (P = 0.188, I2 = 37.4%).
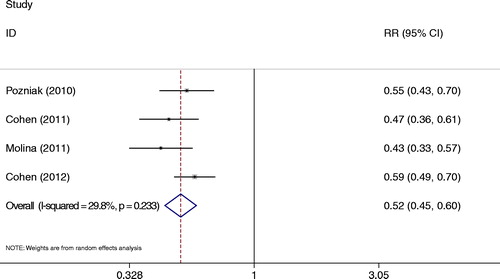
Neurological events
All studiesCitation13–Citation15,Citation19 also revealed neurological events at week 48. Neurological events of interest was at a significantly lower incidence with RPV than with EFV (RR = 0.52, 95% CI: 0.45–0.60) (). In this meta-analysis, there was no heterogeneity (P = 0.239, I2 = 29.8%).
Publication bias: virological response (viral load)
Publication bias was examined with Egger's (P = 0.489) and Begg's tests (P = 0.117). There was no publication bias. Visual inspection of Begg's funnel plots did not show any substantial asymmetry (figure not shown).
Discussion
RPV is the newest member of the class of NNRTIs that inhibits the HIV-1 reverse transcriptase in an allosteric manner.Citation33 It is a potent and selective inhibitor of HIV-1 reverse transcriptase.
The overall meta-analysis results demonstrated that non-inferior antiviral efficacy was observed in viral load comparable with EFV at 48 weeks (P>0.05). At present, viral load has been used as a surrogate marker for these endpoints in clinical trials and in the context of HIV-1 therapy; decreases in plasma viral load exhibit a linear relationship with lower risk of AIDS progression and death.Citation34 The pooled estimate of viral load represents a clinically meaningful decrease in the RPV group.
The median CD4+T-lymphocyte counts also increased for the RPV-treated and EFV-treated groups by week 48; however, no statistically significant difference was noted among treatment groups with regard to CD4+T-lymphocyte counts (P>0.05). There was a statistical difference in the virological failure between these two groups (P < 0.05). It is still a concern, necessitating continued research for new antiretroviral treatment options, such as RPV. Based on the similar efficacy observed with the three dosages, the 25-mg daily dose was further developed and studied in Phase III trials due to its favorable risk: benefit profile.Citation13,Citation35
The results of the meta-analysis showed that common adverse events differ significantly between the RPV group and the EFV group. A significantly lower incidence for RPV versus EFV was observed for the following events: rash (any type; P < 0.01), and neurological adverse events (P < 0.01). The efficacy of EFV is established,Citation36 and it is recommended as the first-choice treatment option for HIV-1-infected patients.Citation37 However, the most common side-effects of EFV are rash and CNS toxic effects, necessitating a regimen switch in some patients.Citation38 RPV's most common adverse effects were psychological in nature, with rash being the second most common adverse effect. However, the frequency of adverse effects was less than that seen in the EFV-treated group.Citation35 RPV had a better tolerability with fewer adverse events and fewer adverse events leading to discontinuation of the regimen.Citation39 Our meta-analysis results suggested that RPV is a valid and safe alternative to EFV in the treatment of anti-retroviralnaive patients infected with HIV-1 and is associated with fewer side effects. In addition, Maggi et al. pointed out that the RPV may exert renal toxic effects. Because RPV may inhibit the amount of tubular secretion of creatinine causing a slight increase in serum creatinine levels and consensual eGFRcreat reduction.Citation40 We will continue to follow the literature in this area. If there were a sufficient number of papers, we will evaluate the association between RPV and the effect of renal function.
There are several limitations to this meta-analysis. First, the main limitation of this review is the limited sample size. There are only 2522 patients of the included trials, so the data could not be extended to all patients with HIV-1-infected patients over the world. However, subgroup analyses of the combined ECHO and THRIVE populations by sex, region, ethnic origin, and hepatitis B and C co-infectionCitation17,Citation28,Citation29 show that the efficacy of RPV and EFV are similar, suggesting broader applicability of these data. Second, the four RCTs had a double-blind, double-dummy design, meaning that patients had to take study treatment twice daily, rather than once daily, although the effect of this design feature on response rates is not known. In addition, the period of intervention of the studies is 48 weeks, so long-term effects and adverse events remain to be seen. Finally, the loss to follow-up in these studies may affect the results. Although most of the loss to follow-up in the four RCTs was balanced across treatment arms, there is still potential for selection bias: the individuals remaining under study may not be representative of the randomized sample.
The heterogeneity of some variables is worthy of note in this meta-analysis sample size (only 2522 patients). On the other hand, the four RCTs trials were conducted in 21 countries. Different ethnic populations may affect these results.
Conclusion
RPV is effective and safe for HIV-1-infected patients. However, only four trials and 2522 patients were included in this meta-analysis, so more patients and higher-quality, longer-intervention randomized controlled trials are still required to clarify the issues of the safety and efficacy of RPV in patients with HIV-1 infection.
Disclaimer statements
Contributors
The authors contributed equally to this work. The authors are solely responsible for the design and conduct of this study, all study analyses, and drafting and editing of the manuscript and its final contents. All authors critically reviewed the manuscript for important intellectual content and approved the final manuscript.
Funding
None.
Conflicts of interest
The authors have no conflicts of interest.
Ethical approval
This paper received ethics committee approval.
References
- Clumeck N, Pozniak A, Raffi F, EACS Executive Committee. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of HIV-infected adults. HIV Med. 2008;9(65-71):65.
- Gazzard BG, Anderson J, Babiker A, et al. British HIV Association Guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9(8):563–608.
- Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral Treatment of Adult HIV Infection Recommendations of the International AIDS Society — USA Panel. JAMA. 2010;304(3):321–333.
- Squibb BM. FDA label for efavirenz capsules and tablets. November 2010. http://packageinsertsbmscom/pi/pi_sustivapdf. Accessed May 5, 2011..
- Pérez-Molina JA. Safety and tolerance of efavirenz in different antiretroviral regimens: results from a national multicenter prospective study in 1,033 HIV-infected patients. HIV Clin Trial. 2002;3(279-286):279.
- Das K, Bauman JD, Clark AD Jr, et al. High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc Natl Acad Sci USA. 2008;105(5):1466–1471.
- Chen X, Zhan P, Li D, De CE, Liu X. Recent advances in DAPYs and related analogues as HIV-1 NNRTIs. Curr Med Chem. 2011;18:359–376.
- Lansdon EB, Brendza KM, Hung M, et al. Crystal structures of HIV-1 reverse transcriptase with etravirine (TMC125) and rilpivirine (TMC278): implications for drug design. J Med Chem. 2010;53(10):4295–4299.
- Azijn H, Tirry I, Vingerhoets J, et al. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother. 2010;54(2):718–727.
- Janssen-Cilag International N.V. EDURANT 25 mg Film-Coated Tablets. Summary of Product Characteristics. August 2012. http://www.medicinesorguk/EMC/medicine/25490/SPC/Edurant+25+mg/. Accessed December 13, 2012..
- Janssen Products. EDURANT® (Rilpivirine). Full Prescribing Information. August 2012. http://www.edurant-infocom/sites/default/files/EDURANT-PI.pdf. Accessed December 13, 2012..
- Goebel F, Yakovlev A, Pozniak AL, et al. Short-term antiviral activity of TMC278 — a novel NNRTI — in treatment-naive HIV-1-infected subjects. AIDS. 2006;20:1721–1726.
- Pozniak AL, Morales-Ramirez J, Katabira E, et al. Efficacy and safety of TMC278 in antiretroviral-naive HIV-1 patients: week 96 results of a phase IIb randomized trial. AIDS. 2010;24(1):55–65.
- Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011;378(9787):229–237.
- Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378(9787):238–246.
- Wilkin A, Pozniak AL, Morales-Ramirez J, et al. Long-term efficacy, safety, and tolerability of rilpivirine (RPV, TMC278) in HIV type 1-infected antiretroviral-naive patients: week 192 results from a phase IIb randomized trial. AIDS Res Hum Retroviruses. 2012;28(5):437–446.
- Hodder S, Arasteh K, de Wet J, et al. Effect of gender and race on the week 48 findings in treatment-naive, HIV-1-infected patients enrolled in the randomized, phase III trials ECHO and THRIVE. HIV Med. 2012;13(7):406–415.
- Nelson M, Amaya G, Clumeck N, et al. Efficacy and safety of rilpivirine in treatment-naive, HIV-1-infected patients with hepatitis B virus/hepatitis C virus coinfection enrolled in the Phase III randomized, double-blind ECHO and THRIVE trials. J Antimicrob Chemother. 2012;67(8):2020–2028.
- Cohen C, Wohl D, Arribas J, et al. STAR study: single-tablet regimen emtricitabine/rilpivirine/tenofovir DF is non-inferior to efavirenz/emtricitabine/tenofovir DF in ART-naive adults. Paper presented at: 11th International Congress on Drug Therapy in HIV Infection; November 11–15, 2012; Glasgow, Scotland..
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64.
- Balamane M, Varghese V, Melikian GL, Fessel WJ, Katzenstein DA, Shafer RW. Panel of prototypical recombinant infectious molecular clones resistant to nevirapine, efavirenz, etravirine, and rilpivirine. Antimicrob Agents Chemother. 2012;56(8):4522–4524.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomised clinical trials; is blinding necessary? Control Clin Trials. 1996;17:1–12.
- Brozek JL, Akl EA, Alonso-Coello P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64(5):669–677.
- Fleiss J. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–145.
- Lau J, Ioannidis J, Schmid C. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826.
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634.
- Rimsky L, Vingerhoets J, Eygen VV, et al. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the Phase 3 ECHO and THRIVE studies: 48-week analysis. J Acquir Immune Defic Syndr. 2012;59(1):39–46.
- Cohen CJ, Molina JM, Cahn P, et al. Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naive HIV-1-infected patients:pooled results from the Phase 3 double-blind randomized ECHO and THRIVE trials. J Acquir Immune Defic Syndr. 2012;60(1):33–42.
- Nelson MR, Elion RA, Cohen CJ, et al. Rilpivirine versus efavirenz in HIV-1-infected subjects receiving emtricitabine/tenofovir DF: pooled 96-week data from ECHO and THRIVE studies. HIV Clin Trials. 2013;14(3):81–91.
- Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27(6):939–950.
- Mills AM, Antinori A, Clotet B, et al. Neurological and psychiatric tolerability of rilpivirine (TMC278) vs. efavirenz in treatment-naive, HIV-1-infected patients at 48 weeks. HIV Med. 2013;14(7):391–400.
- Molina JM, Clumeck N, Redant K, Rimsky L, Vanveggel S, Stevens M. Rilpivirine vs. efavirenz in HIV-1 patients with baseline viral load 100,000 copies/ml or less: week 48 phase III analysis. AIDS. 2013;27(6):889–897.
- Debyser Z, Pauwels R, Andries K, et al. An antiviral target on reverse transcriptase of human immunodeficiency virus type 1 revealed by tetrahydroimidazo-[4,5,1-jk] [1,4]benzodiazepin-2 (1H)-one and -thione derivatives. Proc Natl Acad Sci USA. 1991;88(4):1451–1455.
- Marschner IC, Collier AC, Coombs RW, et al. Use of changes in plasma levels of human immunodeficiency virus type 1 RNA to assess the clinical benefit of antiretroviral therapy. J Infect Dis. 1998;177:40–47.
- James C, Preininger L, Sweet M. Rilpivirine: a second-generation nonnucleoside reverse transcriptase inhibitor. Am J Health Syst Pharm. 2012;69(10):857–861.
- Maggiolo F. Efavirenz: a decade of clinical experience in the treatment of HIV. J Antimicrob Chemother. 2009;64(5):910–928.
- HHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. Jan 10, 2011. http://www.aidsinfo.nih.gov/ContentFiles/Adult and Adolescent GL.pdf. Accessed June 22, 2011..
- Bacheler L, Jeffrey S, Hanna G, et al. Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy. J Virol. 2001;75(11):4999–5008.
- Schrijvers R, Desimmie BA, Debyser Z. Rilpivirine: a step forward in tailored HIV treatment. Lancet. 2011;378(9787):201–203.
- Maggi P, Montinaro V, Rusconi S, et al. The problem of renal function monitoring in patients treated with the novel antiretroviral drugs. HIV Clin Trials. 2014;15(3):87–91.