Abstract
Background and objective:
Worldwide, 50% of human immunodeficiency virus (HIV)-infected people are women. This study was to evaluate whether the safety and efficacy outcomes of three initial antiretroviral regimens (ARVs) differed by sex.
Methods:
Antiretroviral regimen naive participants from nine countries in four continents were assigned to ARVs with efavirenz (EFV) plus lamivudine–zidovudine, atazanavir (ATV) plus didanosine (ddI)-EC/emtricitabine (FTC) or EFV plus FTC–tenofovir–DF. The primary objective was to estimate the sex difference on efficacy outcome of treatment failure defined as one of the following: 1. Time to 1st of confirmed virologic failure, 2. WHO Stage 4 progression or 3. death with hazard ratio (HR) and 95% confidence interval (CI) from adjusted Cox regression models.
Results:
In all, 739 (47%) women and 832 (53%) men with HIV were evaluated. Women had higher pretreatment CD4+(182 vs 165 cells/mm3; P < 0.001) and lower HIV-1 RNA (4.9 log10 vs 5.2 log10 copies/ml; P < 0.001) compared to men. Association of sex with time to regimen failure differed by treatment arm (P = 0.018). For atazanavir plus didanosine-EC plus emtricitabine, women had a longer time to treatment failure compared to men [adjusted HR (aHR) = 0.59; 95% CI 0.40–0.87]. Women were less likely to prematurely discontinue treatment prematurely (aHR = 0.74; 95% CI 0.56–0.98). Women assigned to efavirenz plus lamivudine–zidovudine were more likely to have a primary safety event compared to men (aHR = 1.49; 95% CI 1.18–1.88).
Conclusion:
Antiretroviral efficacy and safety differed by sex in this study. Consideration of potential effects of sex on antiretroviral outcomes is important for the design of future clinical trials and for HIV treatment guidelines.
Introduction
Approximately half of the 35 million people living with human immunodeficiency virus type 1 (HIV-1) are women and the majority live in resource-limited settings (RLS).Citation1 Although women account for a substantial proportion of the global population infected with HIV, they are underrepresented in clinical trials of antiretroviral therapy and current HIV-1 treatment practices are based largely on data from Caucasian male populations.Citation2,Citation3 Women pass through different stages during their lives such as pregnancy and menopause, which could affect drug metabolism and consequently therapeutic response.Citation4,Citation5 Unfortunately, little is known about sex difference associated with responses to antiretroviral therapy, especially for women from diverse racial and ethnic groups in resource limited countries.
Existing studies of whether there are sex-based differences in antiretroviral therapy outcomes have had different conclusions. Some published literature suggest differences between women and men in the pharmacokinetics, efficacy, and safety of antiretroviral therapy.Citation6,Citation7 Post hoc and secondary analyses of other studies have not identified sex-based differences in the efficacy and safety of antiretrovirals.Citation8,Citation9 Other, mainly observational studies have reported a higher frequency of antiretroviral-related adverse effects in women, such as increased risk for lactic acidosis, nevirapine-associated rashes, and fat redistribution.Citation10–Citation15
Additional data from large randomized clinical trials with study populations representative of the worldwide epidemic of HIV-1 infection are needed to better inform guidelines for antiretroviral use in men and women. The objective of this post hoc analysis was to investigate the effects of sex on antiretroviral efficacy and safety, and participant retention in a randomized clinical trial of initial antiretroviral therapy, the Prospective Evaluation of Antiretrovirals in Resource-Limited Settings (PEARLS) study of the AIDS Clinical Trials Group (ACTG study A5175), which a high proportion of women from diverse settings with randomized assignment of antiretroviral regimens (ARVs).Citation16
Methods
Design overview and patient setting/participation
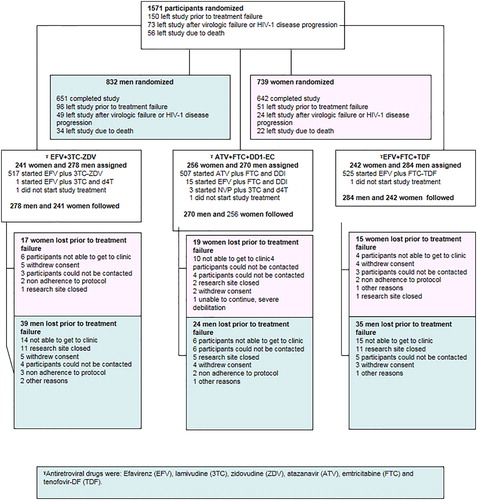
The parent PEARLS study enrolled 1571 antiretroviral-naïve participants with CD4+ lymphocyte count < 300 cells/mm3 from nine countries (Brazil, Haiti, India, Malawi, Peru, South Africa, Thailand, the United States, and Zimbabwe) from May 2005 to August 2007 and followed participants until May 2010.Citation16 Women who received prior single-dose nevirapine or zidovudine for pregnancy on study and prevention mother-to-child transmission (PMTCT) were included. Women who used two or more antiretroviral drugs for PMTCT for more than 7 days within the prior 6 months were excluded. Potential participants were also excluded if they had an acute illness, opportunistic infection with less than 2 weeks of treatment, pregnant, chemotherapy or radiation therapy or a laboratory values > Grade 2 per the DAIDS toxicity Table 2004 within the prior 45 days.Citation17 Informed consent was obtained from all participants, and the human experimentation guidelines of the U.S. Department of Health and Human Services were followed. The study was approved by local site institutional review boards and ethics committees. The CONSORT check list was used in the preparation of this manuscript.
Randomization and intervention
Study participants were randomly assigned with equal probability within country and HIV-1 viral load strata ( < 100 000 copies/ml vs ≥ 100 000 copies/ml) to one of the three open-label ARVs: efavirenz plus co-formulated lamivudine-zidovudine (EFV+3TC-ZDV), atazanavir plus didanosine-EC plus emtricitabine (ATV+DDI-EC+FTC), or efavirenz plus co-formulated emtricitabine-tenofovir-DF (EFV+FTC-TDF). Atazanavir without boosting was used in naïve patients as this was an approved use of Atazanavir during the time the study was conducted.
Outcomes and follow-up
The primary endpoint of the parent PEARLS study was treatment failure defined as the time from randomization to first occurrence of one of the following: (1) virologic failure defined as two successive measurements of plasma HIV-1 RNA ≥ 1000 copies/ml starting at study visit week 16 or later, (2) HIV-1 disease progression; or (3) death due to any cause. The primary safety endpoint was time from randomization to first occurrence of one of the following: (1) onset of first grade ≥ 3 (at least one grade higher than entry) sign/symptom, (2) first laboratory abnormality grade ≥ 3 (at least one grade higher than entry), or (3) last dose of antiretrovirals before regimen change. Hyperbilirubinemia from atazanavir was not considered in the study as a safety endpoint as this is an expected effect of this drug. Participants who did not meet the efficacy or safety endpoint were censored at the earliest of the last study visit that the following occurred: viral load measured, last study visit for safety endpoint assessment or final medication dose. Premature study discontinuation occurred when the last study visit occurred prior to the study close-out period (April–May 2010).Citation16
Study oversight and monitoring
The United States National Institute of Allergy and Infectious Diseases (NIAID) Multinational Data Safety Monitoring Board reviewed safety and efficacy at least yearly. During a routine review (May 22, 2008), the ATV+DDI-EC+FTC regimen was found to be significantly inferior in efficacy compared to EFV+3TC-ZDV, participants receiving ATV+DDI-EC+FTC were placed on an alternative ARV and followed.Citation16
Statistical analysis
A secondary analysis of the primary study was performed to evaluate sex-based comparisons on the primary efficacy, safety, and retention outcomes, and their components. For evaluation of virologic failure on the arm that was stopped early (ATV+DDI-EC+FTC), participants who had not already experienced virologic failure became at risk for this outcome at the 16 weeks visit following initiation of the subsequent ARV, and respective failure/censoring times were calculated from randomization. Distributions of pretreatment/entry characteristics were compared between sexes using Wilcoxon rank sum test for continuous variables and chi-square tests for categorical variables.
Sex differences for time-to-event outcomes were assessed using Cox proportional hazard models stratified by country and screening HIV RNA group. Direction and magnitude of sex differences were estimated with hazard ratios (HRs) and associated two-sided, 95% confidence intervals (CIs). Other covariates, including age at entry, race, ethnicity, pretreatment (screening) CD4+ cell count, entry HIV-1 RNA viral load, weight, body mass index (BMI), AST, ALT, history of antiretroviral use, Karnofsky score, and body measurements were added to the basic model and tested to be retained in the model using a backward selection method (using a significance level of 0.05 to remain in the model), in order to arrive at a multivariable model with adjusted hazard ratios (aHR). In all analyses, treatment arm was included by randomized allocation and analyzed using intent-to-treat principle.
Among women, Fisher's exact test explored the association between treatment failure outcome and each of its three components, between those who had prior antiretroviral experience for PMTCT versus those who did not. Among the subgroup of women with reproductive potential, Fisher's exact test evaluated the association of treatment failure, virologic failure and safety outcome, between women who became pregnant post study entry but before the outcome of interest (and regardless of pregnancy outcome), versus those who did not. Pregnancy absolute incidence rate and relative ratio among categories of entry age, randomized regimen and screening CD4 groups were explored by the event count data model (i.e. Poisson regression). Piecewise (two-phase with knot at week 24) linear longitudinal models of CD4+ cell count levels over time were estimated in order to examine if sex effects on CD4+ counts over time persisted after adjusting for the randomized regimen, pretreatment CD4+ count, and country.
Results
Patient enrollment and follow-up
Of 1571 participants enrolled, 47.0% (N = 739) were women. Women were younger than men (median 33 vs 35 years P < 0.001). Womens’ median pretreatment CD4 count (182 cells/mm3) was higher and HIV plasma HIV-1 RNA (4.9 log10 copies/ml) lower when compared to men (165 cells/mm3; P < 0.001 and 5.2 log10 copies/mL; P < 0.001, respectively). Proportion with a prior or current AIDS defining illness was lower in women (6.6% vs 14.5%; P < 0.001) (see and CitationRef. 18 for more entry characteristics by sex).
Table 1. Entry participant characteristics by sex.
Premature study discontinuation occurred in (9.5%, N = 150/1571) of the participants. The most common reason for leaving the study before treatment failure was not being able to return to clinic (37.3% N = 56/150) (). Women were less likely to prematurely discontinue study treatment (aHR = 0.74; 95% CI 0.56–0.98, adjusted for age, plasma HIV RNA, and Karnofsky score) or study participation (aHR = 0.75; 95% CI 0.56–1.00, adjusted for age, Karnofsky score, and ethnicity) and the risk of premature treatment discontinuation and premature participation discontinuation did not vary significantly by treatment arm (P = 0.70 and 0.90, respectively).
Efficacy outcomes
While approximately 20% of both women and men experienced a treatment failure outcome, this significantly differed among the three treatment groups (interaction P = 0.020; ) Within ATV+DDI-EC+FTC, there was a statistically significant longer time to treatment failure in women compared to men aHR 0.59 (CI 0.40–0.87). However, there was no difference in time to treatment failure within the other two ARV treatment arms ().
Table 2. Efficacy and safety outcomes by sex and treatment arm.
From further evaluation of the three components of treatment failure, women assigned to ATV+DDI-EC+FTC arm had lower and slower rates of virological failure as compared to men (aHR = 0.56; 95% CI 0.36–0.86; ). These estimated effects for virological failure were similar in adjusted models controlling for possible confounding factors including age, screening CD4, and self-reported race (). There was not a significant sex difference (or evidence of sex by treatment interactions) for the two other definitions of treatment failure: time to HIV disease progression or time to death (). There were a relative small number of events for each of these two outcomes.
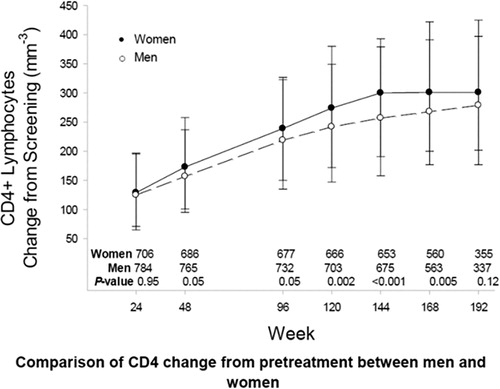
Without adjustment for treatment arm, pretreatment CD4+ count, or country, the estimated (or modeled) mean absolute CD4+ cell counts were 16 cells/mm3 (95% CI 8–25) higher in women compared to men over time. However, when pretreatment CD4+ cell count was added to the model, the sex effect on absolute CD4+ count over follow-up was no longer statistically significant (mean difference between sexes < 1 cell/mm3 per week; P = 0.80; ).
Safety outcomes
For the primary safety outcome, differences by sex varied significantly by treatment arm (interaction P = 0.002). Within the EVF+3TC-ZDV arm, women had a significantly higher rate and shorter time to a primary safety event (aHR = 1.49; 95% CI 1.18–1.88). This effect remained after adjusting for potential confounders such as entry AST value, self-reported ethnic group and anthropometric measurement (). However, in the other two arms, there was not a difference in the primary safety outcome by sex: ATV+DDI-EC+FTC (aHR = 0.82; 95% CI 0.64–1.05), EFV+FTC/TDF (aHR = 0.91; 95% CI 0.70–1.19) (). There was not a difference by sex (or sex by treatment interaction) for either the sign or symptom component (aHR = 1.05; 95% CI 0.82–1.35), or overall laboratory abnormality component (HR = 0.8; 95% CI 0.7–1.1) of the primary safety outcome (). Therefore, the effect in the primary safety outcome appeared to be a result of a sex by treatment difference in the first dose modification component of the outcome (interaction P = 0.060). Adjusting for screening CD4, age, and entry Karnofsky score, within EVF+ZDV-3TC, women were more likely to have a dose modification compared to men (HR = 1.4; 95% CI 1.0–1.8) most likely due to neutropenia caused by ZDV. However, among the other arms, there was not a difference in risk of dose modification by sex EVF+FTC-TDF (HR = 0.9; 95% CI 0.68–1.35)/ATV+DDI-EC+FTC (HR = 0.84; 95% CI 0.62–1.14).
Pregnancy on study and prevention mother-to-child transmission (PMTCT)
Among women with reproductive potential, the incidence of pregnancy was 2.7 per 100 women years (95% CI 2.2–3.4). Women who were randomly assigned to an efavirenz containing study regimen were required to use two forms of birth control, which may have decreased the overall pregnancy incidence in this study. Women were tested for pregnancy at every study visit; therefore, early pregnancy, which may have resulted in miscarriage may have been over-represented in this study. Pregnancy outcomes from this study have been reported elsewhere.Citation19 There was not a significant difference in pregnancy incidence among the three regimen arms (P = 0.38) nor an association between pregnancy and CD4+ cell count (P>0.30). Primary efficacy or primary safety outcomes were not significantly associated with a proceeding pregnancy diagnosis (data not shown). There was no difference in treatment failure between the small subgroup of women who had limited PMTCT (17.2%; 10 of 58), as allowed by the protocol, and those women who did not have PMTCT experience (19.4%; 132 of 681; P = 0.86).
Conclusions
Prospective Evaluation of Antiretrovirals in Resource-Limited Settings evaluated the efficacy and safety of initial antiretroviral therapy in a prospective clinical trial conducted in high-, medium-, and low-income countries in Africa, Asia, the Caribbean, and North and South America. To our knowledge, PEARLS is the only randomized clinical trial to recruit a near equal number of men and women from these diverse cultural, socioeconomic, and geographic settings. The randomized assignment of initial antiretroviral therapy to a large and equal number of women and men in PEARLS provided a unique opportunity to evaluate potential associations between sex and antiretroviral therapy efficacy and safety outcomes. In this context, we found significant differences in both antiretroviral efficacy and safety, and in study retention, between women and men.
Among participants assigned to an ARV of ATV+DDI-EC+FTC, women had decreased risk of treatment failure compared to men. In contrast, the risk of treatment failure did not differ by sex for participants assigned to initial ARVs of efavirenz with either co-formulated lamivudine or emtricitabine-tenofovir-DF. Several previous studies with smaller sample sizes conducted in developed country settings have not shown sex-related differences in immunological and virological outcomes of antiretroviral therapy.Citation20–Citation24 In contrast to the findings in PEARLS, a large randomized study of antiretroviral-naive men and women in the United States (A5202) found that women randomized to ritonavir-boosted atazanavir had poorer efficacy outcomes, with a 2.5 times higher rate of virological failure to atazanavir/ritonavir compared to women taking efavirenz. An important difference between PEARLS and A5202 is that atazanvir was not boosted with ritonavir in PEARLS. Since protease inhibitor clearance may be lower among women,Citation25–Citation27 women in the A5202 study may not have tolerated the adverse effects related higher systemic concentrations of this drug brought about by ritonavir inhibition of atazanavir metabolism.Citation28 Better adherence to the ATV+DDI-EC+FTC regimen among women than men could also explain sex differences in efficacy. An adherence analysis from the PEARLS study demonstrated overall better adherence in women compared to their male counterparts. However, this adherence analysis was not stratified by ARV treatment arms.Citation29 Analysis of plasma antiretroviral drug levels in the PEARLS study have been analyzed (manuscript in press). Pharmacokinetic analysis showed a significant association between sex and ATV C24 where males tended to have a lower C24 estimates compared to females (Adriana Andrade, personal communication, 2014).
A second key finding in PEARLS was that women assigned to a regimen of efavirenz with co-formulated lamivudine–zidovudine had a higher risk of a primary safety outcome and regimen discontinuation, compared to men. Since this safety difference was driven by an increased rate of neutropenia among women, it seems likely that the safety difference may be due to the zidovudine component of this regimen. Sex differences in adverse drug reactions between men and women have been noted previously. Other studies have also found that women are more likely to have lactic acidosis, gastro-intestinal symptoms, and significant drug reactions to nevirapine.Citation8–Citation15,Citation30,Citation31 In PEARLS, sex differences in antiretroviral safety were not detected among participants assigned to either ATV+DDI-EC+FTC or EFV+FTC-TDF.
A sex effect was also observed on retention outcomes in this study, with men having a higher risk of premature study discontinuation or stopping antiretroviral therapy. The most frequent reasons for discontinuation were inability to come to the clinic and loss to follow-up. Other studies in low-income countries have found higher rates of treatment failure in men due to non-adherence and loss to follow-up. An evaluation from the Tanzanian government treatment cohort showed that compared to women, men were 19% more likely to be lost to follow-up HR = 0.74; 95% CI 0.56–0.98).Citation32 Two large studies in Kenya and South Africa also showed that HIV-infected men were more likely to become lost to follow-up and non-adherent than women, both before and after starting antiretrovirals.Citation33,Citation34 Moreover, women are more adapted to daily medication, such as contraceptive pills or iron/folic acid supplementation during pregnancy.Citation35 Alcohol abuse, which is more frequent among African HIV-infected men than women may also be a factor to the significant increase of lost to follow-up in men in this study.Citation36
Interestingly, women participating in North American-based studies were more likely to discontinue study treatment than men. In the GRACE and REALMRK studies, which were powered to specifically evaluate sex differences, women were more likely to discontinue study treatment and follow-up.Citation37,Citation38 The characteristics of the HIV-infected populations in resource-limited and -rich settings are quite different, reflecting patterns of concentrated and generalized epidemics. How cultural, educational, and economic differences might differentially affect retention of women relative to men deserves further investigation.
The strengths of PEARLS for evaluating sex differences in antiretroviral efficacy and safety were the relative and absolute numbers of women enrolled from nine countries in four continents; a median follow-up of 3.8 years (maximum 5 years) with regular visits for adverse events, HIV disease and adherence monitoring; birth control accessibility and a low pregnancy rate. Although sex-based analysis was not the primary objective of the PEARLS and the study was not designed to evaluate sex effects or differences in treatment effect by sex, the analysis present herein was prompted by the statistically significant treatment by sex interactions in the primary analysis.
Limitations of this study were that collection of socio-demographic data such as housing, education, and income level was limited and the lack of this information may have precluded a better understanding of the factors that influence patients’ motivation for study follow-up. While the large absolute and relative sample size of women within this single clinical trial provided an opportunity to explore these associations without the added heterogeneity of combining data across different clinical studies via meta-analyses, statistical power for detecting sex associations with rare outcomes (like AIDS progression or death), or small subgroups within women (i.e. pregnancy or previous antiretroviral exposure for PMTCT) was limited by small numbers of events.
Women have been underrepresented in clinical trials of antiretroviral therapy, and current antiretroviral treatment guidelines are based largely on the clinical trial populations that primarily included male subjects.Citation2,Citation3 The sex discrepancy in clinical trial populations, relative to the global burden of HIV-1 infection, has raised concerns that the evidence base for informing antiretroviral treatment recommendations for women is inadequate. PEARLS provides evidence that these concerns are realistic and both antiretroviral efficacy and safety can differ between men and women. The implications of these findings are twofold: first, researchers must continue to strive to improve the evidence base for antiretroviral safety and efficacy in women. Future clinical trials should be designed to include sufficient numbers of women to define, with greater precision, the risk/benefit ratio for both individual antiretroviral drugs and drug combinations in women. Second, antiretroviral treatment guidelines should be updated to reflect new information from clinical trials with greater numbers of women. Recommendations should take into account that both antiretroviral safety and efficacy can be different in non-pregnant women and sex should influence choice of ARVs.
Acknowledgements
The authors of this paper would like to acknowledge and thank the following site study personal for their work on this A5175 study:
Dr. N. Kumarasamy and Dr. S. Poongulali – YRG CARE Medical Ctr., VHS Chennai CRS (Site 11701) ACTG CTU Grant U01AI069432,
Brenda Hoagland, MD and Rodrigo Escada, MD – Instituto de Pesquisa Clinica Evandro Chagas (Site 12101) ACTG CTU Grant 1U01AI069476,
Dr. Sima Berendes and Dean Soko – College of Med. JHU CRS (Site 30301) ACTG CTU Grant 1U01AI069518,
Ms. Jayinthie Govender and Penelope Madlala – Durban Adult HIV CRS (Site 11201) ACTG CTU Grant 1U01AI069426,
Mina Hosseinipour and Cecilia Kanyama – Lilongwe Clinical Research Site (Site 12001) ACTG CTU Grant UAI069423B,
Thira Sirisanthana, MD and Daralak Tavornprasit, MSc – Chiang Mai University ACTG CRS (Site 11501) ACTG CTU Grant 2UM1AI069399-08 REVISED,
Marineide Gonçalves de Melo, MD and Rita Alves Lira, MD – Hospital Conceicao, Porto Alegre Brazil (Site 12201) ACTG CTU Grant AI 069401-01,
Alexandra Apollon and Marie Josee Joseph, RN – Les Centres GHESKIO CRS (Site 30022) ACTG CTU Grant 1U01AI069421-08,
Dr. Srikanth Tripathy – Nari Pune CRS (Site 11601) ACTG CTU Grant UM1AI069417,
Jorge Sanchez, MD, MPH and Alberto La Rosa, MD – Asociacion Civil Impacta Salud y Educacion. Barranco CRS (Site 11301) ACTG CTU Grant 2UM1AI069438-08,
Rosa Infante, MD and Fanny Garcia, RN – San Miguel CRS (Site 11302) ACTG CTU Grant AI069438,
Mamta Jain, MD and Tianna Petersen, MS – UT Southwestern Medical Center at Dallas (Site 3751) Grant 3U01AI046376-05S4,
Carl J. Fichtenbaum, MD and Michelle Saemann, RN – University of Cincinnati CRS (Site 2401) ACTG CTU Grants 1U01AI069513-01 and 1-UM1AI69439,
Dr. Raman Gangakhedkar – Gadikhana CRS, Pune (Site 11602) ACTG CTU Grant UM1AI069417,
Michael Para, MD and Laura Laughlin, RN – The Ohio State Univ. AIDS CRS (Site 2301) ACTG CTU Grant 1U01AI069474,
Donna McGregor and Baiba Berzins – Northwestern Univ. CRS (2701) ACTG CTU Grant 5U01-AI-0694,
Lisa Kessels RN, ACRN, and Michael Royal BS, RPh – Washington University in St. Louis CRS (Site 2101) ACTG CTU Grant AI 69439-08,
Donna Mildvan, MD and Tessa Gomez, MD – Beth Israel Med. Ctr. ACTU (Site 2851) ACTG CTU Grant 1U01AI46370,
Karen Tashima, MD and Helen Patterson, LPN – The Miriam Hospital (Site 2951) ACTG CTU Grant 2UM1AI069472-08,
P. Jan Geiseler, MD and Bartolo Santos, RN – University of Southern California CRS (Site 1201) ACTG CTU Grant AI069428,
Eric Daar, MD and Ruben Lopez, MD – Harbor-UCLA (Site 603) ACTG CTU Grant A1069424; UL1TR000124,
David Currin, RN, CCRC, ACRN and Catherine Kronk, BA – UNC Global CTU: Chapel Hill CRS (Site 3201) ACTG CTU Grant UM1 AI069423-08; CTSA Grant 1UL1TR001111; CFAR Grant P30 AI50410,
Vicki Bailey, RN and Becky Basham Vanderbilt Therapeutics Clinical Research Site (Site 3652) ACTG CTU Grant 2UM1AI069439-08,
Pablo Tebas, MD and Carol DiGiorgio, RN – Hosp. of the Univ. of Pennsylvania CRS (Site 6201) Grant UM1-AI069534-08; CFAR Grant 5-P30-AI-045008-15,
Michael T Yin and Madeline Torres – Columbia Physicians and Surgeons CRS (Site 30329) ACTG CTU Grant 2UM1-AI069470-08; CTSA 5UL1 RR024156,
Janice Fritsche and Beverly Sha, MD – Rush CRS (Site 2702) ACTG CTU Grant UM1AI069471,
William A. O'Brien MD, MS and Gerianne Casey – UTMB at Galveston (Site 6301) ACTG CTU Grant AI32782,
Judith Currier, MD and Maria Palmer – UCLA CARE Center (Site 601) ACTG CTU Grant UM1 AI069424,
Dr. Oluwatoyin Adeyemi – Cook County Hosp CORE Ctr. (Site 2705),
Todd Stroberg, RN and Luis Lopez-Detres, BA – Cornell Clinical Research Site (Site 7804) ACTG CTU Grant UM1AI069419; CTSC Grant UL1TR000457.
Disclaimer Statements
Contributors All authors participated in the writing, of the review of the data results of the manuscript. The core/initial writing team consisted of CF, TBC, LSM, BG. LSM performed the statistical analysis. CF, BG,UL,SF, WS,RI, AR, NK, JH, TBC participated in the implementation of the study. TBC, BG,LMS, JH,NG, were involved in the initial study design.
Funding The project was supported by Award Number UM1AI068636 NIAID and by National Institute of Mental Health (NIMH) and National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAIDS or the National Institutes of Health. Employees of the NIAID participated as study team members provided recommendations on the study and approved the final study design. They had no role in data collection and analysis, decision to publish, or preparation of the manuscript. Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline provided study drug and Gilead Sciences purchased study drug that was not available. This work was supported by the Statistical and Data Management Center of the AIDS Clinical Trials Group, under the National Institute of Allergy and Infectious Diseases grant No. 1 U01 AI068634.
ClinicalTrials.gov identifier: NCT00084136
Conflicts of interest Three authors have declaration of COI, none which influence the outcome of this analysis. Ms Laura Smeaton worked as a consultant for Pfizer within a different population and disease. Dr Thomas Campbell is a consultant for Gilead and Dr Umesh Lalloo has either active or pending grants with Astra—Zeneca, Cipla and Rambaxy.
Ethics approval Informed consent was obtained from all participants and the human experimentation guidelines of the U.S. Department of Health and Human Services were followed. The study was approved by local site institutional review boards and ethics committees. CONSORT check list was used in the preparation of this manuscript.
References
- UNAIDS Global Report. http://www.unaids.org/en/resources/documents/2014/HIV_estimates_with_uncertainty_bounds_1990-2013. Accessed November 26, 2014..
- d'Arminio Monforte A, González L, Haberl A, Sherr L, Ssanyu-Sseruma W, Walmsley SL, et al. Better mind the gap: addressing the shortage of HIV-positive women in clinical trials. AIDS. 2010;24:1091–1094.
- The American College of Obstetricians and Gynecologists. Gynecologic care for women with human immunodeficiency virus. Practice Bulletin Number 117. Obstet Gynecol. December 2010;116:1492–1509.
- Isoherranen N, Thummel KE. Drug metabolism and transport during pregnancy: how does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab Dispos. 2013;41:256–262.
- Harris RZ, Benet LZ, Schwartz JB. The effects of menopause and hormone replacement therapies on prednisolone and erythromycin pharmacokinetics. Clin Pharmacol Ther. 1996;59:429–435.
- Currier JS, Spino C, Grimes J, Wofsy CB, Katzenstein DA, Hughes MD, et al. Differences between women and men in adverse events and CD4+ responses to nucleoside analogue therapy for HIV infection. The AIDS Clinical Trials Group 175 Team. J Acquir Immune Defic Syndr. 2000;24:316–324.
- Clark R. Sex differences in antiretroviral therapy-associated intolerance and adverse events. Drug Saf. 2005;28:1075–1083.
- Hoffman RM, Umeh OC, Garris C, Givens N, Currier JS. Evaluation of sex differences of fosamprenavir (with and without ritonavir) in HIV-infected men and women. HIV Clin Trials. 2007;8:371–380.
- Da Silva B, Cohen D, Gibbs S, Fredrick L, Bernstein B. Impact of gender on response to lopinavir/ritonavir (LPV/r) tablets dosed QD or BID administered with tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) in antiretroviral-naıve (ARV) subjects: Results from study M05-730. Presented at: 17th International AIDS Conference, August 3–8, 2008; Mexico City, Mexico. [Poster TUPE0069]..
- Eluwa GI, Badru T, Akpoigbe KJ. Adverse drug reaction to antiretroviral therapy [ARV]: incidence, type and risk factors in Nigeria. BMC Clin Pharmacol. 2012;12:1472–6904.
- Dong BJ, Zheng Y, Hughes MD, Frymoyer A, Verotta D, Lizak P, et al. Nevirapine pharmacokinetics and risk of rash and hepatitis among HIV-infected sub-Saharan African women. AIDS. 2012;26:833–841.
- Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, et al. Severe hepatotoxicity associated with nevarapine use in HIV-infected subjects. J Inf Dis. 2005;191:825–829.
- Wester CW, Eden SK, Shepherd BE, Bussmann H, Novitsky V, Samuels DC, et al. Risk factors for symptoms hyperlactemia and lactic acidosis among combination antiretroviral therapy-treated adults in Botswana: results from a clinical trial. Aids Res Hum Retroviruses. 2012;28:759–765.
- Castelnuovo B, Kiragga A, Kamya MR, Manabe Y. Stavudine toxicity in women is the main reason for treatment change in a 3-year prospective cohort of adult patients started on first- line antiretroviral treatment in Uganda. J Acquir Immune Defic Syndr. 2011;56:59–63.
- Squires KE. Gender differences in the diagnosis and treatment of HIV. Gend Med. 2007;4:294–307.
- Campbell TB, Smeaton LM, Kumarasamy N, Flanigan T, Klingman KL, Firnhaber C, et al. A randomised clinical trial of the efficacy and safety of once-daily antiretroviral therapy for initial treatment of HIV-1 infection in diverse multinational settings: the ACTG PEARLS Study. PLoS Med. 2012;9:e1001290.
- Division of AIDS (DAIDS). Table for grading the severity of adult and pediatric adverse events. DAIDS RSC; 2004. http://rsc.tech-res.com/safetyandpharmacovigilance/.
- Grinsztejn B, Smeaton L, Barnett R, Klingman K, Hakim J, Flanigan T, et al. Sex-associated differences in pre-antiretroviral therapy plasma HIV-1 RNA in diverse areas of the world vary by CD4 (+) T-cell count. Antivir Ther. 2011;16:1057–1062.
- Nielsen-Saines K, Komarow L, Cu-Uvin S, Jourdain G, Klingman KL, Shapiro DE, et al. Assessment of safety and toxicity following maternal antiretroviral exposure in infants born to HIV-infected women enrolled in antiretroviral treatment protocols in diverse areas of the world. Eighteen month results of AIDS Clinical Trials Group [ACTG] Study 5190/Pediatric AIDS Clinical Trials Group (PACTG) 1054. Pediatrics. 2012;129:e1525–e1532.
- Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE Study. J Acquir Immune Defic Syndr. 2010;53:323–332.
- Moore AL, Kirk O, Johnson AM, Katlama C, Blaxhult A, Dietrich M, et al. Virologic, immunologic, and clinical response to highly active antiretroviral therapy: the gender issue revisited. J Acquir Immune Defic Syndr. 2003;32:452–461.
- Prins M, Meyer L, Hessol NA. Sex and the course of HIV infection in the pre- and highly active antiretroviral therapy eras. AIDS. 2005;19:357–370.
- Nicastri E, Angeletti C, Palmisano L, Sarmati L, Chiesi A, Geraci A, et al. Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. AIDS. 2005;19:577–583.
- Nicastri E, Leone S, Angeletti C, Palmisano L, Sarmati L, Chiesi A, et al. Sex issues in HIV-1-infected persons during highly active antiretroviral therapy: a systematic review. J Antimicrob Chemother. 2007;60:724–732.
- Sekar V, Ryan R, Schaible D, Mazikewich A, Mrus J. Pharmacokinetic profile of darunavir (DRV) co-administered with low dose ritonavir in treatment experienced women and men: 4 week analysis in a substudy of the GRACE trial. Presented at: 9th International Workshop on Clinical Pharmacology of HIV Therapy (IWCPHIV); April 7–9, 2008; New Orleans, LA..
- Burger DM, Siebers MC, Hugen PW, Aamoutse RE, Hekster YA, Koopmans PP. Pharmacokinetic variability caused by gender: do women have higher indinavir exposure than men? J Acquir Immune Defic Syndr. 2002;29:101–102.
- Fletcher CV, Jiang H, Brundage RC, Acosta EP, Haubrich R, Katzenstein D, et al. Sex-based differences in saquinavir pharmacology and virologic response in AIDS Clinical Trials Group Study 359. J Infect Dis. 2004;189:1176–1184.
- Smith KY, Tierney C, Mollan K, Venuto CS, Budhathoki C, Ma Q, et al. Outcomes by sex following treatment initiation with atazanavir plus ritonavir or efavirenz with abacavir/lamivudine or tenofovir/emtricitabine. Clin Infect Dis. 2014;58:555–563.
- Safren SA, Biello KB, Smeaton L, Mimiaga MJ, Walawander A, Lama JR, et al. Psychol social Predictors of non-adherence and treatment failure in a large scale multi-national trial of antiretroviral therapy for HIV: Data from the ACTG A5175/PEARLS trial. Plos One. 2014;9:e104178.
- Squires KE, Johnson M, Yang R, Uy J, Sheppard L, Absalon J, et al. Comparative gender analysis of the efficacy and safety of atazanavir/ritonavir at 96 weeks in the CASTLE study. J Antimicrob Chemother. 2011;66:363–370.
- Kumarasamy N, Venkatesh KK, Cecelia AJ, Devaleenol B, Saghayam S, Yepthomi T, et al. Gender-based differences in treatment and outcome among HIV patients in South India. J Womens Health. 2008;17:1471–1475.
- Hawkins C, Chalamilla G, Okuma J, Spiegelman D, Hertzmark E, Aris E, et al. Gender differences in antiretroviral treatment outcomes among HIV-infected adults in Dar es Salaam, Tanzania. AIDS. 2011;25:1189–1197.
- Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika AM, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010;88:681–688.
- Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43:78–84.
- Johannessen A. Are men the losers of the antiretroviral treatment scale-up? AIDS. 2011;25:1225–1226.
- Farley J, Miller E, Zamani A, Tepper V, Morris C, Oyegunle M, et al. Screening for hazardous alcohol use and depressive symptomatology among HIV-infected patients in Nigeria: prevalence, predictors, and association with adherence. J Int Assoc Physicians AIDS Care. 2010;9:218–226.
- Currier J, Averitt Bridge D, Hagins D, Zorrilla CD, Feinberg J, Ryan R, et al. Sex-based outcomes of Darunavir-Ritonavir therapy: The GRACE (gender, race, and clinical experience) study. Ann Intern Med. 2010;153:349–357.
- Squires KE, Bekker LG, Eron JJ, Cheng B, Rockstroh JK, Marquez F, et al. Safety, tolerability, and efficacy of raltegravir (RAL) in a diverse cohort of HIV-infected patients (pts): 48 week results from the REALMRK study. AIDS Res Hum Retroviruses. 2013;29:859–870.