Abstract
Objective:
A therapeutic vaccination based on a synthetic peptide (AT20) representative of the HIV-1 matrix protein p17 (p17) functional region, coupled to keyhole limpet hemocyanin (KLH) AT20-KLH was capable of inducing the production of high-avidity antibodies (Abs) toward a previous untargeted p17 hotspot of functional activity in highly active antiretroviral therapy (HAART)-treated HIV-1-infected patients. Since avidity of Abs after immunization and the retention of antigens are important in sustaining the long-lasting production of specific humoral responses, we asked whether AT20-KLH vaccination would result in development of a long-lived immune response.
Methods:
The long-term duration of Ab response to AT20-KLH has been evaluated in 10 patients previously enrolled for the AT20-KLH vaccination trial at day 898 post-immunization. Ab titer and their avidity was assessed using specifically designed ELISA assays, whereas their neutralizing capacity was estimated in vitro using a ‘wound sealing assay’.
Results:
Data obtained show that high titers of specific anti-AT20 Abs were maintained at more than 2 years after the last immunization. Furthermore, these Abs were capable to neutralize exogenous p17, as assessed by ability of sera derived from AT20-KLH-immunized patients to block the ability of p17 to promote cell migration in vitro.
Conclusion:
This finding attests for a successful AT20-KLH vaccine molecule formulation and for an effective HAART-dependent Ab persistence.
Introduction
HIV-1 matrix protein p17 (p17) exhibits different immunomodulatory and chemokine-like properties, which may be relevant in the context of viral pathogenesis.Citation1 All p17 activities are mediated by its binding to specific cellular receptors,Citation2,Citation3 through a functional epitope located at the p17 NH2-terminal region.Citation4 Antibodies (Abs) directed toward the p17 functional region were neutralizing all p17 biological activities by displacing the binding of viral protein to its cellular receptors.Citation5,Citation6 The occurrence of Ab response to the p17 functional region during the natural course of HIV-1 infection is rare and eventually, at a very low titer.Citation1 Thus, despite the presence of high-titer anti-p17 Abs, sera from HIV-1-infected patients were not usually found to neutralize the biological activity of viral protein.Citation1 On the basis of these evidences and successful preclinical data,Citation7 a 20 amino acids-long synthetic peptide (AT20) representative of the p17 functional region, coupled to the carrier protein keyhole limpet hemocyanin (KLH) was selected as the active agent to perform a therapeutic phase I clinical trial in highly active antiretroviral therapy (HAART)-treated HIV-1-infected patients. All tested AT20-KLH doses were safe and well tolerated. Moreover, all vaccinated patients developed high titers of high-avidity p17 neutralizing Abs.Citation8 These findings provided the first evidence that the AT20 peptide-based approach was successful in its aim of redirecting HIV-1-infected patients' humoral responses toward a previous untargeted hotspot of functional activity.
Avidity of Abs after immunization as well as antigen retention in lymph node germinal centers are known to correlate with the long-lasting production of specific humoral responses.Citation9,Citation10 Indeed, p17 is not only easily detected in blood of patientsCitation11 but it has been also demonstrated that it accumulates and persists in abundant amounts in germinal centers of patients' lymph nodes before and after containment of HIV-1 by therapy.Citation12 Consisting with such findings, we designed a study to prove whether immunization with AT20-KLH would result in development of a long-lived immune response sustained by a potent humoral immune response and by a continuous natural exposure to p17.
Methods
This is a single-center prospective observational study where we enrolled all the patients followed up at the Clinic of Infectious and Tropical Diseases of Brescia who participated at the therapeutic phase I study MED-AT20-001, (EudraCT n.2008-001465-29) a multi-center, randomized, dose escalation clinical trial. For a thorough explanation of the study protocol and inclusion criteria, please refer the main study article.Citation8 Briefly, the patients were HIV-1-infected, clinically asymptomatic individuals, in HAART therapy for at least 1 year, HIV-1 viral load < 50 copies/ml for at least 6 months and CD4+T-cell count ≥ 350 cells mm− 3 for at least 3 months prior to inclusion at entry, and CD4 nadir ≥ 200 cell mm− 3. They were randomized 3: 1 to receive AT20-KLH vaccine, obtained conjugating the GMP-grade AT20 peptide (OPC, Heidelberg, Germany) with the GMP-grade KLH (Byosin, CA, USA) as carrier protein. The patients in the treatment arm were then randomized 1: 1 to receive via intramuscular injection either 25 μg (Arm A) or 100 μg (Arm B) of vaccine. The patients subsequently attended our outpatient services with a schedule of 3–6 months, up to 24 months (day 898) after the study conclusion (day 168): at every visit, standard laboratory tests including CD4+T-cell count, HIV–RNA, basic hematology, and biochemistry were performed. After that, the patients underwent clinical examination, and adverse events (AEs) and concomitant medications were recorded. Finally, antiretroviral therapy was evaluated and modified according to patients' needs. Blood samples were collected at day 898, and detection of specific Ab and Ab avidity index were performed by specifically developed ELISA assays as already described.Citation8 Neutralization capability of Abs developed following AT20-KLH vaccination was assessed by impairing the p17 pro-migratory activity on human umbilical vein endothelial cells (HUVECs), as already described.Citation3
Results and Discussion
Ten out of the 24 total study patients were followed up in our outpatient clinic, 7 of whom received the AT20-KLH. In particular, six were in the Arm A (25 μg for each dose) and one was in the Arm B (100 μg for each dose) of vaccine; 3 belonged to the control arm. Seven out of 10 patients were males and median age was 43 (range 23–59) years. Median CD4+T-cell count at nadir was 347 cells μl− 1 (range 243–500 cells μl− 1). We recorded only two AEs occurring from day 168 to day 898 and unrelated to the vaccine administration: one patient was hospitalized for acute gastroenteritis and another patient had a mild elevation of serum aminotransferase of unknown origin. This data demonstrated a good long-term safety of the AT20-KLH vaccine. All patients remained virologically suppressed throughout the 2 years of observation, and no virological blips were recorded. Moreover, no significant differences in the trend of CD4+T-cell count, CD4% and CD4/CD8 ratio were observed over the 2-year long observation period ().
Table 1. Trends of CD4+T-cell count. CD4% and CD4/CD8 ratio upon AT20-KLH immunization.
As previously described,Citation8 patients enrolled in the MED-AT20-001 trial did not show consistent levels of anti-AT20 Abs at day 0 (Ab titer < 10 in 6/7 patients belonging to Arm A and Arm B of the study; Ab titer = 100 in 1/7 patients) whereas 100% of immunized subjects developed high titers of AT20-specific Abs by the end of vaccination (day 168). Notably, at day 898, anti-AT20 Abs were consistently maintained in all patients immunized with AT20-KLH. In fact, comparison of Ab titers between day 168 and day 898 post-immunization showed only a modest fold reduction in anti-AT20 Ab levels [median fold decrease: 2.5 (range: 1–5)]. The same trend was observed also when Abs to the entire p17 protein were evaluated. At day 0, 6/7 immunized subjects had negligible or low levels of anti-p17 Abs (Ab titer < 10 in 3/7 patients; Ab titer ≤ 100 in 3/7 patients) and AT20-KLH vaccination consistently increased anti-p17 Ab levels in all of them. Again, titers of anti-p17 Abs induced by AT20-KLH vaccination were long lasting [anti-p17 titer median fold decrease at day 898 as compared to day 168: 1 (range: 1–3)]. Patients belonging to the control group displayed no detectable anti-AT20 and anti-p17 at day 0 and day 168, as well as at day 898 (Ab titer < 10). This result attests for an inability of these HIV+ patients to develop Abs to the p17 functional epitope AT20 in the absence of AT20-KLH immunization ().
Figure 1. Changes in Ab titers over time after AT20-KLH immunization. Levels of anti-AT20 Abs were evaluated by ELISA, using plates coated with unconjugated AT20 peptide (A) or with the entire recombinant p17 protein (B). Ab titers detected in sera obtained at day 0, at the end of immunization protocol (day 168) and 2 years later (day 898) are shown. Each sign represents data obtained from a single AT20-KLH-immunized subject or from a control patient. A value of 1 was arbitrarily assigned to each Ab titer of < 10.
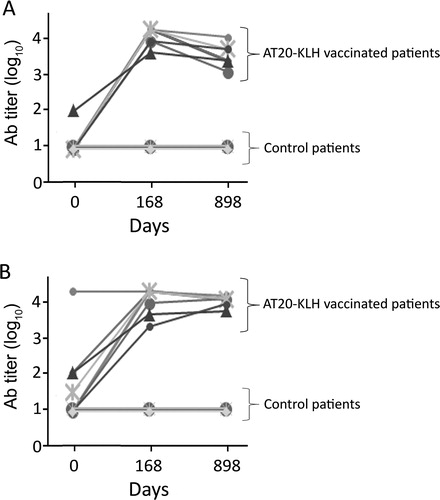
Finally, it is worth to note that at day 898 all patients showed high-avidity AT20 Abs (avidity grade ≥ 3).
Although the number of patients in the study was small, we also sought to evaluate if Ab titers detected at day 898 in AT20-vaccinated patients correlate with the laboratory parameters evaluated at the same time point. When data were analyzed by Pearson test, no correlation was observed between Ab titer and absolute CD4+T-cell count, CD4% and/or CD4/CD8 ratio.
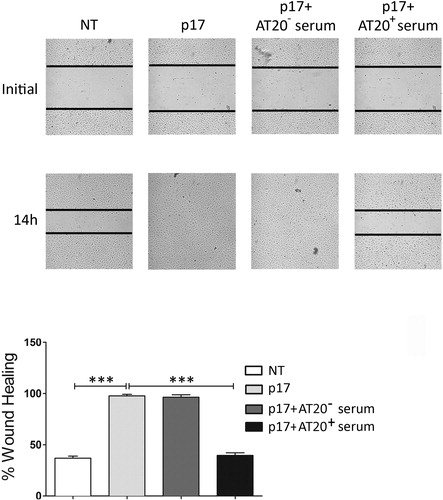
As shown in , sera obtained from vaccinated patients – but not sera obtained from control patients – neutralized the capability of p17 to promote human endothelial cell migration, which occurs following the interaction between viral protein and the chemokine receptors CXCR1 and CXCR2.Citation3
Figure 2. P17-driven migration of human endothelial cells is inhibited by sera obtained from AT20-KLH-vaccinated patients. In the wound-healing assay, confluent HUVEC monolayers were scratched using a 200 μl pipette tip then treated with medium alone (NT), p17 (10 ng ml− 1), or p17 (10 ng ml− 1) previously incubated for 30 minutes at 4°C with decomplemented serum (1: 50 dilution) obtained from an AT20-KLH-immunized patient (AT20+ serum) or from a not vaccinated patient (AT20− serum). Cells were observed by optical microscopy and, after 14 hours of incubation, pictures were taken (original magnification 10 × ). Images are representative of three independent experiments with similar results. Bar graph reports the percentage of wound repair observed upon different experimental conditions. Values are the mean ± SD of the three experiments performed. Statistical analysis was calculated using one-way ANOVA and Bonferroni post-test was used to compare data: ***P < 0.001.
This study points to a strong immunogenicity of the AT20-KLH molecule. This is probably due, at least in part, to the characteristic of the synthetic peptide. The AT20 peptide has been designed as a long (20-mer) peptide to be degraded by proteolytic enzymes and presented exclusively by professional APCs, thereby ensuring sufficient co-stimulation. In fact, shorter peptides can be directly loaded on any MHC molecule, also on non-professional APCs, which may lead to the induction of tolerance.Citation13 Also, the modality of cross-linking the non immunogenic AT20 peptide to the carrier protein KLH may have played a critical role in conferring a strong immunogenicity to the vaccine molecule. In fact, maleimide conjugation was preferred to the glutaraldehyde one, since maleimide conjugation has been previously shown to strongly enhance peptide KLH conjugates' immunogenicity as compared to glutaraldehyde.Citation14 Finally, a strong adjuvant for peptide vaccination as Montanide, was chosen over other adjuvants for its capability to form a depot at the site of injection, leading to ‘leakage’ of antigen into the bodyCitation15 and then to rapidly dissolve so avoiding tolerance.Citation16 Taken together, the success of AT20-KLH in 100% of HAART-treated patients in redirecting a long-lasting humoral response toward a previously untargeted p17 hotspot of functional activity is likely to reside in the design of the vaccination strategy as a whole. It is worth noting that the first and most successful peptide-based vaccine that is currently licensed is a therapeutic vaccine against human papillomavirus, capable of inducing long-lasting vaccine-specific immune responses in all immunized patients. Definitely, it contains long synthetic peptides directed against viral oncoproteins, mixed in Montanide.Citation17
Our study confirms also the capability of HAART to fully restore patient's humoral response. From our data it emerge that phenotypic and functional B-cell abnormalities commonly observed in untreated HIV-1-infected patients are completely restored by HAARTCitation18 in order to achieve the development of such a perfect functioning of anti-AT20 Ab producing memory B-cells over time. Serological memory is linked to the quality of Abs obtained during vaccination. Several reports support the evidence that Ab avidity after immunization is a good correlate of the development and maintenance of memory B-cell responses,Citation9,Citation10 and indeed, all vaccinated patients developed high-avidity Abs to AT20 at the end of the p17-based AT20-KLH therapeutic immunization.Citation8 Previous studies have established that p17 is trapped and persists in patients' lymph node germinal centers before and after containment of HIV-1 by therapy.Citation10 It is worth noting that the germinal center is a dynamic microenvironment, where antigen-bound follicular dendritic cells help B-cells in maintaining serological memory.Citation19,Citation20 Therefore, the capability of patients successfully treated with HAART to generate a long-lived humoral response to the p17 functional epitope in the absence of detectable viremia, may be due to really effective memory B-cell responses to AT20 combined with a persistent immune system activation by long-term retention of extracellular p17 in the lymph node germinal centers.
In conclusion, it is likely that the generation of long-lasting neutralizing Abs to a previously untargeted p17 functional epitope is the consequence of an optimal formulation of the AT20-KLH vaccine molecule, of a successful HAART-dependent recovery of B-cell immune functions and of the retention of p17 in lymph node germinal centers. Our experience may prove useful in formulating new immunization strategies aimed to stimulate the quality and persistence of humoral responses.
Acknowledgments
We thank Medestea Research & Production S.p.A., Turin, Italy for kindly providing us with the AT20 synthetic peptide.
Disclaimer Statements
Contributors
EF, SF, FC, and AC conceived and designed the study. SF, FC, and AC obtained funding and/or ethics approval. EF and MI collected, analyzed, and interpreted the data. FC, DM, and CG collected and analyzed results. EF, MI, SF, and AC wrote the article.
Funding
The therapeutic phase I study MED-AT20-001 was sponsored by Medestea Research & Production S.p.A. This study was supported by Institutional Research Grants from the University of Brescia.
Conflicts of interest
Emanuele Focà received speakers' honoraria and grants for participating to Conferences from Bristol-Myers Squibb, Merck-Sharp & Dohme, Gilead Sciences, Viiv Healthcare, Abbvie, Janssen. Francesco Castelli acts as Principal Investigator in clinical trials sponsored by BMS, ViiV, Janssen Cilag, Roche, MSD, and Novartis. He has received research grants from Pfizer, Viiv, Astellas, and Abbott. Arnaldo Caruso and Simona Fiorentini are inventors in patents owned by Medestea. All the other authors declare no conflicts of interest.
Ethics approval
The study protocol was approved by the institutional ethic committee and was conducted in accordance with the ICH guidelines on Good Clinical Practice and the Helsinki/EdinburghDeclaration. Written informed consent was obtained from each participant.
References
- Fiorentini S, Giagulli C, Caccuri F, Magiera AK, Caruso A. HIV-1 matrix protein p17: a candidate antigen for therapeutic vaccines against AIDS. Pharmacol Ther. 2010;128:433–444.
- Giagulli C, Magiera AK, Bugatti A, Caccuri F, Marsico S, Rusnati M, et al. HIV-1 matrix protein p17 binds to the IL-8 receptor CXCR1 and shows IL-8-like chemokine activity on monocytes through Rho/ROCK activation. Blood. 2012;119:2274–2283.
- Caccuri F, Giagulli C, Bugatti A, Benetti A, Alessandri G, Ribatti D, et al. HIV-1 matrix protein p17 promotes angiogenesis via chemokine receptors CXCR1 and CXCR2. Proc Natl Acad Sci U S A. 2012;109:14580–14585.
- De Francesco MA, Baronio M, Fiorentini S, Signorini C, Bonfanti C, Poiesi C, et al. HIV-1 matrix protein p17 increases the production of proinflammatory cytokines and counteracts IL-4 activity by binding to a cellular receptor. Proc Natl Acad Sci U S A. 2002;99:9972–9977.
- Fiorentini S, Marini E, Bozzo L, Trainini L, Saadoune L, Avolio M, et al. Preclinical studies on immunogenicity of the HIV-1 p17-based synthetic peptide AT20-KLH. Biopolymers. 2004;76:334–343.
- Fiorentini S, Marini E, Caracciolo S, Caruso A. Functions of the HIV-1 matrix protein p17. New Microbiol. 2006;29:1–10.
- Fiorentini S, Marsico S, Becker PD, Iaria ML, Bruno R, Guzmán CA, et al. Synthetic peptide AT20 coupled to KLH elicits antibodies against a conserved conformational epitope from a major functional area of the HIV-1 matrix protein p17. Vaccine. 2008;26:4758–4765.
- Iaria ML, Fiorentini S, Focà E, Zicari S, Giagulli C, Caccuri F, et al. Synthetic HIV-1 matrix protein p17-based AT20-KLH therapeutic immunization in HIV-1-infected patients receiving antiretroviral treatment: a phase I safety and immunogenicity study. Vaccine. 2014;32:1072–1078.
- Fried AJ, Altrich ML, Liu H, Halsey JF, Bonilla FA. Correlation of pneumococcal antibody concentration and avidity with patient clinical and immunologic characteristics. J Clin Immunol. 2013;33:847–856.
- Alam MM, Arifuzzaman M, Ahmad SM, Hosen MI, Rahman MA, Rashu R, et al. Study of avidity of antigen-specific antibody as a means of understanding development of long-term immunological memory after Vibrio cholerae O1 infection. Clin Vaccine Immunol. 2013;20:17–23.
- Fiorentini S, Riboldi E, Facchetti F, Avolio M, Fabbri M, Tosti G, et al. HIV-1 matrix protein p17 induces human plasmacytoid dendritic cells to acquire a migratory immature phenotype. Proc Natl Acad Sci U S A. 2008;105:3867–3872.
- Popovic M, Tenner-Racz K, Pelser C, Stellbrink HJ, van Lunzen J, Lewis G, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2005;102:14807–14812.
- Bijker MS, Van Den Eeden SJ, Franken KL, Melief CJ, Van Der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol. 2008;38:1033–1042.
- Kafi K, Betting DJ, Yamada RE, Bacica M, Steward KK, Timmerman JM. Maleimide conjugation markedly enhances the immunogenicity of both human and murine idiotype-KLH vaccines. Mol Immunol. 2009;46:448–456.
- Bijker MS, Van Den Eeden SJ, Franken KL, Melief CJ, Offringa R, Van Der Burg SH. CD8+ CTLpriming by exact peptide epitopes in incomplete Freund's adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;178:5033–5040.
- Welters MJ, Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, et al. Multiple CD4 and CD8 T-cell activation parameters predict vaccine efficacy in vivo mediated by individual DC-activating agonist. Vaccine. 2007;25:1379–1389.
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar epithelial neoplasia. N Engl J Med. 2009;361:1838–1847.
- Fogli M, Torti C, Malacarne F, Fiorentini S, Albani M, Izzo I, et al. Emergence of exhausted B cells in asymptomatic HIV-1-infected patients naïve for HAART is related to reduced immune surveillance. Clin Dev Immunol. 2012;2012:829584.
- Haberman AR, Schlomchik MJ. Reassessing the function of immune-complex retention by follicular dendritic cells. Nat Rev Immunol. 2003;3:757–764.
- Kosco-Vilbois MH. Are follicular dendritic cells really good for nothing? Nat Rev Immunol. 2003;3:764–769.
