Abstract
Objectives
The objective of the study is to evaluate the possible roles of the detection of circulating B cells secreting anti-glycoprotein IIb/IIIa antibody, platelet glycoprotein IIb/IIIa, and anti-glycoprotein IIb/IIIa antibody in the diagnosis of primary immune thrombocytopenia (ITP) patients.
Methods
Circulating B cells secreting anti-glycoprotein IIb/IIIa antibody, platelet glycoprotein IIb/IIIa and anti-glycoprotein IIb/IIIa antibody in 64 patients with ITP, 33 non-ITP patients, and 32 controls were measured with enzyme-linked immunospot assay (ELISPOT), monoclonal antibody immobilization of platelet antigens assay (MAIPA) and flow cytometic analysis (FCM), respectively.
Results
Compared with the controls and non-ITP patients, the frequency of circulating B cells secreting anti-glycoprotein IIb/IIIa antibody was significantly increased, whereas the positive rate of platelet glycoprotein IIb/IIIa was significantly decreased (P < 0.05) in ITP patients, respectively. The sensitivities for the diagnosis of ITP of ELISPOT and FCM were 68.8% and 57.8%, and the specificities of 90.9% and 90.9%, respectively. The sensitivities of ELISPOT and FCM were higher than MAIPA's sensitivity (39.1%) (P < 0.05). However, there was no apparent difference of the sensitivities of ELISPOT and FCM and the specificities of those three detections (MAIPA's specificity was 81.8%) (P > 0.05).
Discussion
ELISPOT and FCM for detecting the circulating B cells secreting anti-glycoprotein IIb/IIIa antibody and the platelet glycoprotein IIb/IIIa were as specific as that of MAIPA for assay of anti-glycoprotein IIb/IIIa antibody, but ELISPOT and FCM had higher sensitivities. So ELISPOT and FCM were sensitive and specific for identifying patients with autoantibody-mediated thrombocytopenia and these should be used as diagnostic tests in clinic.
Introduction
Primary immune thrombocytopenia (ITP) is an acquired immune-mediated disorder characterized by isolated thrombocytopenia and mucocutaneous bleeding, that is, the results of increased platelets destruction or decreased platelet production or a combination of both.Citation1–Citation4 The classical viewpoint considers that ITP is caused by platelet autoantibodies. The antibodies are recognized by Fcγ receptors expressed on macrophages that reside in the spleen and elsewhere, and are cleared more rapidly.Citation5,Citation6 Platelet clearance may be a more important mechanism than impaired marrow platelet production that may also be a result of thrombopoiesis inhibition by the platelet antibodies.Citation7–Citation9 Recently, the cytotoxic effects of CD8+ T lymphocytes have been proven to cause thrombocytopenia in some patients with ITP, perhaps by impairing megakaryocytopoiesis.Citation10–Citation12
The concept of the pathophysiology of ITP has therefore shifted from the traditional view of increased platelet consumption mediated by autoantibodies to more complex mechanisms, such as impaired production of platelet and T-cell-mediated immune effects. However, platelet autoantibodies produced by autoreactive B cells against platelet self-antigens, especially immunoglobulin (IgG) antibodies against glycoprotein IIb (GP IIb)/IIIa and or GP Ib/IX, are still considered to play a crucial role in the pathogenesis of ITP.Citation13–Citation17 These autoantibodies bind to circulating platelets, leading to platelet destruction by the reticuloendothelial system. Anti-glycoprotein (GP) IIb/IIIa antibodies, which were shown to be detected in majority chronic ITP patients when sensitive assays were used, were one of the primary autoantibodies found in patients with ITP.Citation18–Citation20
The enzyme-linked immunospot assay (ELISPOT) assay is a standard technique for detecting individual B lymphocytes that secrete specific antibodies.Citation21 Circulating B cells secreting anti-GP IIb/IIIa antibody in the peripheral blood of ITP patients are mostly memory B cells released from the spleen and those autoreactive B cells reflect the autoantibody response in the spleen.Citation22 The preliminary studies showed that the ELISPOT assay for detecting circulating B cells secreting anti-GP IIb/IIIa antibody was a sensitive and specific method for identifying patients with autoantibody-mediated thrombocytopenia.Citation22–Citation24
The symptoms and signs in ITP are highly variable and range from completely asymptomatic to hemorrhage from any site, with intracranial bleed the most serious.Citation25 The diagnosis of ITP remains one of exclusion of other causes of isolated thromobocytopenia using patient history, physical examination, blood count, and evaluation of the peripheral blood film. There is no gold standard test that can reliably establish the diagnosis. How to accurate diagnosis of ITP is still a challenging problem. In this study, we analyzed for circulating B cells secreting anti-GP IIb/IIIa antibody, platelet GP IIb/IIIa, and anti-GP IIb/IIIa antibody in ITP and non-ITP patients, to assess their potential significances in the differential diagnosis of ITP.
Materials and methods
Patients
Patients and the healthy controls in this study were enrolled from the Department of Hematology and the Medical Examination Center of the Second Hospital of Shanxi Medical University. Informed consent was obtained from each patient and healthy control in accordance with the Declaration of Helsinki. Ethical approval for the study was obtained from the Medical Ethical Committee of the Second Hospital of Shanxi Medical University. Enrolled into this study were 64 patients with ITP, 33 non-ITP patients, and 32 healthy controls. Of the 64 ITP patients, 33 were newly diagnosed (19 female and 14 male, median 37 years old), 31 had persistent and chronic ITP (18 female and 15 male, median 40 years old). All patients with ITP included in this study were diagnosed according to the international consensus guidelines.Citation26 The 33 non-ITP patients consisted of 15 aplastic anemia, 15 myelodysplastic syndrom (MDS) and 3 acute leukemia (20 female and 13 male, median 40 years old) patients. The 32 healthy controls included 18 female and 14 male with a median age of 39 years. All patients had received no therapy and no platelet transfusion for at least 1 month prior to sampling.
Reagents
Reagents for MAIPA and ELISPOT were CD41 (Beckman Coulter, Fullerton, CA, USA), Goat Anti-Mouse IgG (BD PharMingen, San Diego, CA, USA), polyvinylidene difluoride-bottomed 96-well multi-tier plates (Millipore, Bedford, MA), anti-Human IgG (Fc-specific)-alkaline phosphatase antibody, PNPP, and BCIP/NBT (Sigma-Aldrich, St Louis, MO, USA). FCM was performed using anti-CD61, anti-CD41 monoclonal antibodies (mAbs) (Beckman Coulter) conjugated to FITC or PE. The results were analyzed on a EPICS ELITE ESP flow cytometer (BD PharMingen) using EXPO32 software.
Sample preparation
Platelet-rich plasma (PRP), used for the flow cytometic analysis of platelet GP IIb/IIIa, was prepared from heparinized venous blood by centrifugation, followed by separation into platelets and platelet-poor plasma (PPP). PPP was used for the detection of anti-GP IIb/IIIa antibody. The remaining cell components were subjected to lymph prep density gradient centrifugation to isolate the peripheral blood mononuclear cells (PBMCs). PRP was also prepared by centrifugation from EDTA venous blood. PRP and PBMCs were used fresh, whereas PPP was stored at −20°C until used.
Detection of B cells secreting anti-GP IIb/IIIa antibody
B cells secreting anti-GP IIb/IIIa antibody were detected and quantified using the ELISPOT carried out as previously described in detail.Citation22,Citation23 Briefly, polyvinylidene difluoride-bottomed 96-well multi-tier plates (Millipore, Bedford, MA, USA) were coated with 30 µg/ml of purified human GP IIb/IIIa (Beckman Coulter) and subsequently blocked with 1% bovine serum albumin. PBMCs (105 cells/well) in complete medium were incubated in the GP IIb/IIIa-coated plates at 37°C in a humidified atmosphere of 5% CO2 for 4 hours. After washing away the cells, the membranes were incubated with anti-human IgG (Fc-specific)-alkaline phosphatase antibody (Sigma-Aldrich), and antibodies bound to the membrane were visualized as spots by incubation with BCIP/NBT (Sigma-Aldrich). The number of spots was counted under a dissecting microscope. Each experiment was conducted in four independent wells, and the results represent the mean of the four values. The frequency of B cells secreting anti-GP IIb/IIIa antibody was expressed as the number per 105 PBMC. The cutoff value was set at 5 SD above the mean of the healthy controls.
Detection of platelet GP IIb/IIIa
PRP fixed 0.1% paraformaldehyde 30 minutes, followed by the addition of 5 µl of CD61-PE and CD41-FITC. To PRP from control was added 5 µl of IgG-PE and IgG-FITC and incubated at room temperature protected from light for 30 minutes. Subsequently, the mixture was centrifuged and the cell pallet washed with physiological saline then reconstituted in each 500 µl of physiological saline. The final mixtures were analyzed on the flow cytometer for percent positivity for CD41+/CD61−, CD41−/CD61+, and CD41+/CD61+ using EXPO32 software ().
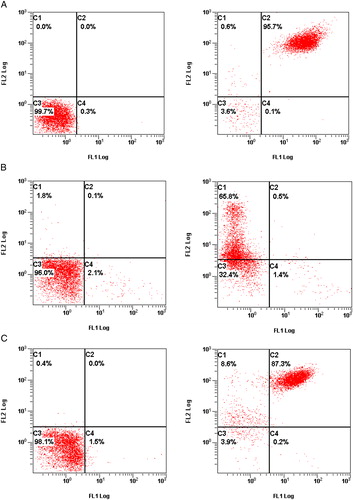
Figure 1. The expression of the GP IIb/IIIa (CD41/CD61) in ITP, non-ITP patients and healthy control, showed as the flow cytometric diagram: in the left figure, the C3 quadrant demonstrated the platelet gated by flow cytometry; in the right figure, the C1, C2, C3, and C4 quadrant represented the percentage of CD41−/CD61+, CD41+/CD61+, CD41−/CD61−, and CD41+/CD61−, respectively. (A) The expression of GPIIb/IIIa (CD41/CD61) in one of the healthy controls: platelet were gated 99.7% by flow cytometry; the percentage of CD41+/CD61−, CD41−/CD61+, and CD41+/CD61+ was 0.1, 0.6, and 95.7%, respectively. (B) The expression of GPIIb/IIIa (CD41/CD61) in one of the ITP patients: platelets were gated 96.0% by flow cytometry. The percentage of CD41+/CD61−, CD41−/CD61+, and CD41+/CD61+ was 1.4, 65.8, and 0.5%, respectively. (C) The expression of the GPIIb/IIIa (CD41/CD61) in one of the non-ITP patients: platelets were gated 98.1% by flow cytometry. The percentage of CD41+/CD61−, CD41−/CD61+, and CD41+/CD61+ was 0.2, 8.6, and 87.3%, respectively.
Detection of anti-GP IIb/IIIa antibody
The detection of anti-GP IIb/IIIa antibody was performed according to the MAIPA described by Hou et al.Citation27 Briefly, the 96-well multi-tier plates were coated with 3 µg/ml of Goat Anti-Mouse IgG (BD PharMingen) for 4°C overnight and subsequently blocked with 0.01 M PBS/Tween/3% bovine serum albumin. Then the coated wells were added 4 µg/ml of purified human GP IIb/IIIa (Beckman Coulter) and incubated for 1 hours. The PRP and platelet (1 × 109 /ml) mixture were incubated at room temperature for 1 hour. After lysing the platelet, the GP IIb/IIIa-coated plates were incubated with the supernatant for 1 hour. Subsequently, the plates were incubated with the anti-human IgG (Fab-specific)-alkaline phosphatase antibody (Sigma-Aldrich), and the optical density (OD)450 were measured after incubation with PNPP (Sigma-Aldrich). All samples were tested in duplicate, and the results were calculated as the duplicate mean. The cutoff level was set at 3 SD above the mean of the healthy controls.
Statistical analysis
Analysis was largely descriptive, based on mean ± standard deviation as well as medians and ranges. Analysis of variance was used to analyze the differences in continuous variables between different groups. P value less than 0.05 was considered as statistically significant. The receiver operator characteristic curve (ROC) and the discriminative validity were calculated by SPSS software. All analyses were carried out using SPSS 13.0 software (SPSS Inc., Chicago, USA).
Results
Detection of the frequencies of circulating B cells secreting anti-GP IIb/IIIa antibody
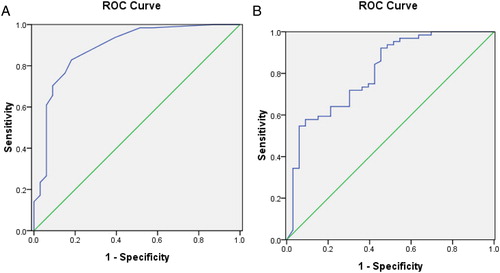
The platelet count of the controls (222.6 ± 45.1 × 109/l) was significantly different from that of patients with ITP (30.8 ± 22.8 × 109/l) and non-ITP (39.8 ± 20.9 × 109/l) but there was no difference between the two thrombocytopenic groups. The frequencies of circulating B cells secreting anti-GP IIb/IIIa antibody were significantly increased (6.5 ± 4.1/105 per PBMC) in ITP patients (P < 0.05), compared with the controls (1.2 ± 0.5/105 per PBMC) and non-ITP patients (2.2 ± 2.0/105 per PBMC). However there was no apparent difference between the controls and non-ITP patients (P > 0.05) (). The frequencies of circulating B cells secreting anti-GP IIb/IIIa antibody in newly diagnosed ITP patients (7.6 ± 4.6/105 per PBMC) were notably increased, compared with the persistent and chronic ITP patients (5.3 ± 3.0/105 per PBMC) (P < 0.05) (). The ROC curve showed the discriminative validity of ELISPOT in the ITP and non-ITP patients was 0.885 ().
Figure 2. The frequencies of circulating B cells secreting anti-GP IIb/IIIa antibody and the expression of the GP IIb/IIIa (CD41/CD61) in ITP, non-immune thrombocytopenia patient and health control determined by ELISPOT and FCM. (A) The frequencies of circulating B cells secreting anti-GP IIb/IIIa antibody in different group. Each experiment was conducted in four independent wells, and the results represent the mean of the four values. The cutoff value which was set at 5 SD above the mean of the healthy controls, was shown by a line (3.7/105 PBMC). (B) The expression of the GP IIb/IIIa (CD41/CD61) in different group. The cutoff value, which was set at the best sensitivity and specificity dot in ITP patients’ ROC curve, was shown by a line (43%).
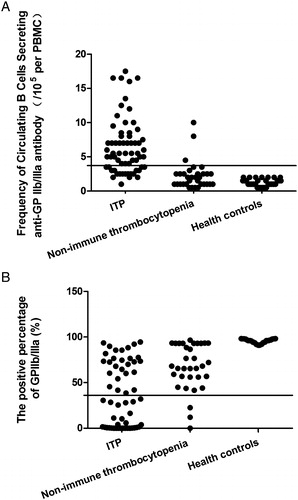
Figure 3. Comparison of the different assays for the diagnosis of ITP between different patient groups and controls. (A) Platelet count in different patient and control groups. (B) Frequency of circulating B cells secreting anti-GP IIb/IIIa antibody. (C) Platelet GP IIb/IIIa (D) anti-GP IIb/IIIa antibody.
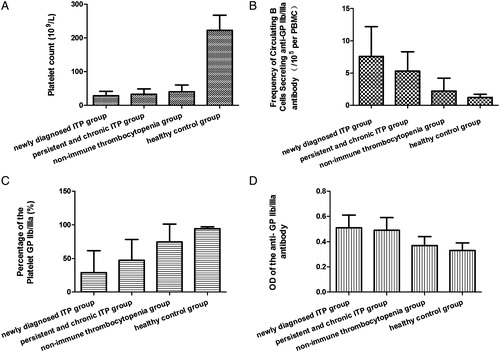
Figure 4. The ROC of the frequency of circulating B cells secreting anti-GP IIb/IIIa antibody and the platelet GP IIb/IIIa in ITP patients and non-immune thrombocytopenia patients. (A) Circulating B cells secreting anti-GP IIb/IIIa antibody: the area under ROC curve is 0.885, the standard error is 0.039, 95% confidence interval is 0.810, 0.961. (B) Platelet GPIIb/IIIa: the area under ROC curve is 0.807, the standard error is 0.047, 95% confidence interval is 0.715, 0.900.
Detection of positive percentage of the platelet GP IIb/IIIa
In the three groups, the positive percentage of CD41 (GPIIb) had no apparent difference (P > 0.05). The ITP and non-ITP patients had higher expression of CD61 (GPIIIa) (P < 0.05). However, there was no significant difference between the thrombocytopenia groups (P > 0.05). GP IIb/IIIa (CD41+/CD61+) were notably decreased in ITP (37.92 ± 32.89%) and non-ITP patients (74.43 ± 26.72%), compared with the controls (94.32 ± 2.70%). However, GP IIb/IIIa (CD41+/CD61+) were significantly different between the two thrombocytopenia group of patients (P < 0.05) (). The expression of GP IIb/IIIa in newly diagnosed ITP patients (29.04 ± 32.66%) was notably decreased compared with that of the persistent and chronic ITP patients (47.37 ± 30.89%) (P < 0.05) (). The ROC curve showed the discriminative validity of positive expression of GP IIb/IIIa in the ITP and non-ITP patients was 0.807 ().
Detection of the OD of the anti-GP IIb/IIIa antibody
Anti-GP IIb/IIIa antibodies in ITP patients were significantly increased (0.50 ± 0.10) (P < 0.05) compared with the controls (0.33 ± 0.06) and non-ITP patients (0.37 ± 0.07). However, the anti-GP IIb/IIIa antibodies were similar between the controls and non-ITP patients, and between the newly diagnosed (0.51 ± 0.10) and persistent and chronic ITP patients (0.49 ± 0.10) (P > 0.05) ().
Diagnostic value comparisons of the three results
ELISPOT detection of the frequencies of circulating B cells secreting anti-GP IIb/IIIa antibody for the diagnosis of ITP had a sensitivity of 68.8%, a specificity of 90.9%, positive likelihood ratio (+LR) of 7.56 and positive predictive value of 93.6%. Choosing the best sensitivitic and specificitic dot in ITP patients’ ROC curve, this was 43%, as the border of diagnosis. Flow cytometry detection of the positive percentage of platelet GP IIb/IIIa for the diagnosis of ITP had a sensitivity of 57.8%, a specificity of 90.9%, positive likelihood ratio (+LR) of 6.36 and positive predictive value of 92.5%. MAIPA detection of anti-GP IIb/IIIa antibody for the diagnosis of ITP had a sensitivity of 39.1%, a specificity of 81.8%, positive likelihood ratio (+LR) of 2.15 and positive predictive value of 80.7 %. Compared with the MAIPA, the ELISPOT (χ2 = 7.53) and flow cytometry (χ2 = 4.03) had higher sensitivities (P < 0.05). However, there was no difference between the ELISPOT and flow cytometry for the diagnosis of ITP (χ2 = 2.12, P > 0.05) ().
Table 1. The circulating B cells secreting anti-GP IIb/IIIa antibody, platelet GP IIb/IIIa and anti-GP IIb/IIIa antibody in ITP patients and non-ITP patients
Discussion
ITP is a heterogeneous autoimmune disease. In addition to the crucial role of humoral immune abnormalities,Citation28 a number of cellular immune disorders have been demonstrated to be involved in the pathogenesis of ITP.Citation29–Citation31 The diagnosis of ITP is made by exclusion of secondary causes of thrombocytopenia as there are no diagnostic tests to confirm ITP.Citation26 Autoantibodies against platelet antigens were considered as one of the important factors in the diagnosis of ITP. Platelet-associated IgG(PAIgG) and platelet antigen-specific antibody assays are available methods to detect antiplatelet autoantibodies. The PAIgG assay has high sensitivity for ITP, but its specificity is low, because the PAIgG is elevated both in immune and non-ITP.Citation32,Citation33
Antigen-specific assays, such as MAIPA (enzyme-linked immunosorbent assay, ELISA and modified antigen capture ELISA test, MACE), have been developed to measure autoantibodies that recognize one or more platelet surface glycoproteins, including GP IIb/IIIa, GPIb/IX and GPIa/IIa, among others. In prospective study, the detection of these platelet-specific autoantibodies has an estimated sensitivity from 49 to 66%, an estimated specificity is 78–92%.Citation34,Citation35 In our study, the sensitivity of MAIPA assay for anti-GP IIb/IIIa antibody is 39.06% and the specificity is 81.82%, similar to the published reports. However, whole a positive antigen-specific assay provides confirmatory evidence for the diagnosis of ITP, a negative test cannot be used to rule out the diagnosis.Citation36
Failure to detect antibodies might reflect limited test sensitivity, undetectable antigens or other mechanisms of platelet destruction.Citation37 In order to find the more sensitive and specific assay, we selected ELISPOT and FCM to detect the frequencies of circulating B cells secreting anti-GP IIb/IIIa antibody and platelet GP IIb/IIIa in this study.
Recent study demonstrates that the activation of autoreactive T and B cells to GP IIb/IIIa induces pathogenic anti-GP IIb/IIIa antibody production.Citation22 ELISPOT assay, which has been extensively applied to the diagnosis of autoimmune disease, for example, tuberculosis,Citation38 has several unique features. These include the ability to detect a single cell of 105 cells, significantly reflection of ongoing antibody production and detection of the antibody producing B cells without interference by binding to the antigen.Citation39 In the preliminary study, they demonstrated that the frequency of circulating B cells secreting anti-GP IIb/IIIa antibody was significantly increased in ITP patients and with a high estimated sensitivity of 84 to 90 percent, and estimated specificity of 90% for the diagnosis of ITP.Citation22,Citation24,Citation40 B cells secreting anti-GP IIb/IIIa antibody in the peripheral blood of ITP patients are mostly memory B cells that are released from the spleen after activation through the antigen-specific interaction. These auto-reactive B cells reflected the ongoing autoantibody response in the spleen.Citation22 In our study, we found the auto-reactive B cells secreting anti-GP IIb/IIIa antibody in the peripheral blood. In addition, the assay could be used to discriminate the different subgroup of ITP patients. Thus, ELISPOT assay to detect the frequency of circulating B cells secreting anti-GP IIb/IIIa antibody may be useful for the diagnosis of ITP.
The GP IIb/IIIa complex (CD41/61), which is the most abundant platelet cell-surface protein, is a receptor for fibrinogen, von Willebrand factor, fibronectin, and vitronectin that are essential for platelet aggregation.Citation41 The most commonly identified antigenic targets of the platelet autoantibodies in ITP is GP IIb/IIIa. The fragments of GP IIb/IIIa, exposed to the autoreactive T and B cells, stimulate the production of autoantibodies that enhance the destruction of platelet.Citation42 Thus, the expression of platelet GP IIb/IIIa could indirectly reflect the pathogenesis of ITP. FCM demonstrated that the expression of GPIIb/IIIIa in patients with ITP was decreased due to autoantibodies binding. This GP IIIb/IIIIa expression was difference between the different stage of ITP.
The primary limitation of this manuscript is the relatively small sample. A second limitation is the lack of patient follow-up. Finally, GP IIb/IIIa and GP Ib/IX are both important proteins expressed on the surfaces of megakaryocytes and platelet but our study only assayed for the GP IIb/IIIa antibody and antigen. Thus, our data should be considered preliminary, awaiting further confirmatory studies on a larger number of patients. We also need to assay for GP Ib/IX antibody and antigen.
In conclusion, compared with the MAIPA assay for measuring anti-GP IIb/IIIa antibody, the ELISPOT assay for detecting the frequency of circulating B cells secreting anti-GP IIb/IIIa antibody and flow cytometry for assessing the percentage of platelet GP IIb/IIIa had higher sensitivity but with equal specificity. In the future, after confirmatory research, the two assays could potentially be used diagnostically for the identification subgroups of ITP with a potential to facilitate individualized treatment selection.
Authorship
Lin-hua YANG contributed to design, coordinated the research, and wrote the manuscript; Jian-fang CHEN performed the research, analyzed data and wrote the manuscript; Jian-fang CHEN, Jian-jun FENG, Li-xian CHANG and Jun-qing LIU recruited patients and coordinated their sample collection and MAIPA study; Jian-fang CHEN performed the analysis of B cells secreting anti-GP IIb/IIIa antibody; Li-xian CHANG performed platelet GP IIb/IIIa detection. All authors read and approved the final version of the manuscript.
Acknowledgements
This work was supported by grants from Key Clinical Research Project of Public Health Ministry of China 2008–2010, Commonweal Trade for Scientific Research (200802031).
References
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346(13):995–1008.
- Cooper N, Bussel J. The pathogenesis of immune thrombocytopenic purpura. Br J Haematol. 2006;133(4):364–74.
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Crowther MA, et al. The American Society 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207.
- Stasi R, Provan D. Management of immune thrombocytopenic purpura in adults. Mayo Clin Proc. 2004;79(4):504–22.
- Ballem PJ, Segal GM, Stratton JR, Gernsheimer T, Adamson JW, Slichter SJ. Mechanisms of thrombocytopenia in chronic autoimmune thrombocytopenic purpura. Evidence of both impaired platelet production and increased platelet clearance. J Clin Invest. 1987;80(1):33–40.
- McMillan R. Autoantobodies and autoantigens in chronic immune thrombocytopenia purpura. Semin Hematol. 2000;37(3):239–48.
- Nugent D, McMillan R, Nichol JL, Slichter SJ. Pathogenesis of chronic immune thrombocytopenia: increased platelet destruction and/or decreased platelet production. Br J Haematol. 2009;146(6):585–96.
- Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102(3):887–95.
- McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103(4):1364–9.
- Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, et al. Improved regulatory T cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639–45.
- Zhang F, Chu X, Wang L, Zhu Y, Li L, Ma D, et al. Cell-mediated lysis of autologous platelets in chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 2006;76(5):427–31.
- Olsson B, Andersson PO, Jernås M, Jacobbson S, Carlsson B, Carlsson LM, et al. T-cellmediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9(9):1123–4.
- Hou M, Stockelberg D, Kutti J, Waldenvik H. Antibodies against GPIb/IX, GPIIb/IIIa, and other platelet antigens in chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 1995;55(5):307–14.
- Mehta YS, Pathare AV, Badakere SS, Ghosh K, Mohanty D. Influence of auto-antibody specificities on the clinical course in patients with chronicand acute ITP. Platelets. 2000;11(2):94–8.
- Fabris F, Scandellari R, Ruzzon E, Randi ML, Luzzato G, Girolami A. Plateletassociated autoantibodies as detected by a solidphase modified antigen capture ELISA test (MACE) are a useful prognostic factor in idiopathic thrombocytopenic purpura. Blood. 2004;103(12):4562–4.
- van Leeuwen EF, van der Ven JT, Engelfriet CP, von dem Borne AE. Specificity of autoantibodies in autoimmune thrombocytopenia. Blood. 1982;59(1):23–6.
- He R, Reid DM, Jones CE, Shulman NR. Spectrum of Ig classes, specificities, and titers of serum antiglycoproteins in chronic idiopathic thrombocytopenic purpura. Blood. 1994;83(4):1024–32.
- Woods VL, Oh EH, Mason D, McMillan R. Autoantibodies against the platelet glycoprotein IIb/IIIa complex in patients with chronic ITP. Blood. 1984;63(2):368–75.
- Fujisawa K, Tani P, McMillan R. Plateletassociated antibody to glycoprotein IIb/IIIa from chronic immune thrombocytopenic purpura patients often binds to divalent cation-dependent antigens. Blood. 1993;81(5):1284–9.
- Chan H, Moore JC, Finch CN, Warkentin TE, Kelton JG. The IgG subclasses of platelet associated autoantibodies directed against platelet glycoproteins IIb/IIIa in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2003;122(5):818–24.
- Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65(1–2):109–21.
- Kuwana M, Okazaki Y, Kaburaki J, Kawakami Y, Ikeda Y. Spleen is a primary site for activation of glycoprotein IIb-IIIa-reactive T and B cells in patients with immune thrombocytopenic purpura. J Immunol. 2002;168(7):3657–82.
- Kuwana M, Okazaki Y, Kaburaki J, Ikeda Y. Detection of circulating B cells secreting platelet-specific autoantibody is useful in the diagnosis of autoimmune thrombocytopenia. Am J Med. 2003;114(4):322–5.
- Satoh T, Pandey JP, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y, et al. Single nucleotide polymorphisms of the inflammatory cytokine genes in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;124(6):796–801.
- Cohen YC, Djulbegovic B, Shamai-Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Int Med. 2000;160(11):1630–8.
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–86.
- Hou M, Peng J, Shi Y, Zhang C, Qin P, Zhao C, et al. Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur J Haematol. 2003;70(6):353–7.
- McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44 (4 Suppl 5):S3–S11.
- Sakakura M, Wada H, Tawara I, Nobori T, Sugiyama T, Sagawa N, et al. Reduced CD4+CD25+ T cells in patients with idiopathic thrombocytopenic purpura. Thromb Res. 2007;120(2):187–93.
- Yu J, Heck S, Patel V, Leva J, Yu Y, Bussel JB, et al. Defective circulating CD25+ regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;112(4):1325–8.
- Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147–50.
- Mueller-Eckhardt C, Kayser W, Mersch-Baumert K, Mueller-Eckhardt G, Breidenbach M, Kugel HG, et al. The clinical significance of platelet-associated IgG: a study on 298 patients with various disorders. Br J Haematol. 1980;46(1):123–31.
- Kelton JG, Powers PJ, Carter CJ. A prospective study of the usefulness of the measurement of platelet-associated IgG for the diagnosis idiopathic thrombocytopenic purpura. Blood. 1982;60(4):1050–3.
- Brighton TA, Evans S, Castaldi PA, Chesterman CN, Chong BH. Prospective evaluation of the clinical usefulness of an antigen-specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenia. Blood. 1996;88(1):194–201.
- Warner MN, Moore JC, Warkentin TE, Santos AV, Kelton JG. A prospective study of protein-specific assays used to investigate idiopathic thrombocytopenic purpura. Br J Haematol. 1999;104(3):442–7.
- Raife TJ, Olson JD, Lentz SR. Platelet antibody testing in idiopathic thrombocytopenic purpura. Blood. 1997;89(3):1112–4.
- Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511–21.
- Wang JY, Chou CH, Lee LN, Hsu HL, Jan IS, Hsueh PR, et al. Diagnosis of tuberculosis by an enzyme-linked immunospot assay for interferon-gamma. Emerg Infect Dis. 2007;13(4):553–8.
- Sedgwick JD. ELISPOT assay: a personal retrospective. Methods Mol Biol. 2005;302:3–14.
- Kuwana M, Kurata Y, Fujimura K, Fujisawa K, Wada H, Nagasawa T, et al. Preliminary laboratory based diagnostic criteria for immune thrombocytopenic purpura: evaluation by muti-center prospective study. J Thromb Haemost. 2006;4(9):1936–43.
- Phillips DR, Charo IF, Parise LV, Fitzgerald LA. The platelet membrane glycoprotein IIb/IIIa complex. Blood. 1988;71(4):831–43.
- Kuwana M, Kaburaki J, Kitasato H, Kato M, Kawai S, Kawakami Y, et al. Immunodominant epitopes on glycoprotein IIb-IIIa recognized by autoreactive T cells in patients with immune thrombocytopenic purpura. Blood. 2001;98(1):130–9.