Abstract
CD25 (interluekin-2 receptor) expression in diffuse large B-cell lymphoma (DLBCL) cells has been not examined. To characterize CD25+ DLBCL, 123 patients, who were newly diagnosed with DLBCL, were analyzed by single-color flow cytometry (FCM). CD25-positivity was significantly higher in DLBCL patients (n = 123; mean ± SD, 27.8 ± 30.6%) than in those with reactive lymphadenopathy (n = 16; mean ± SD, 8.6 ± 4.3%) and follicular lymphoma (n = 60; mean ± SD, 12.7 ± 12.4%). By two-color FCM, CD25/CD19 or CD25/CD20 dual positivity in DLBCL patients was shown: mean ± SD, 63.7 ± 25.5% (n = 13) and 55.0 ± 28.1% (n = 14), respectively. Eighty-two percent of the patients with DLBCL received rituximab combined with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy. A cut-off value of 60% with CD25-positivity clearly divided patients with DLBCL into two groups: CD25-high or CD25-low DLBCL. Although clinical and immunophenotypic features were not significantly different in both groups, the former showed a significantly poorer response and more inferior progression-free survival than the latter. CD25 may be a new prognostic marker and could be a therapeutic target in DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common histologic subtype of non-Hodgkin lymphoma, and is regarded as a heterogeneous group of diseases with varied morphologic, immunophenotypic, and molecular features.Citation1 Several randomized controlled trials have established the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) regimen as standard chemotherapy for DLBCL, potentially curing more than half of patients with DLBCL. However, approximately 20–50% of patients with DLBCL experience treatment failure and a poor outcome following salvage therapy.Citation2–Citation4 Therefore, identifying prognostic markers to individualize therapeutic approaches for DLBCL is important to improve survival.
Interleukin-2 (IL-2) is the growth factor for activated T or B lymphocytes and stimulates the clonal expansion and maturation of these lymphocytes.Citation5 IL-2 mediates its biologic effects by binding to the IL-2 receptor (IL-2R) complex. CD25 (alpha chain of IL-2R) constitutes IL-2R with two different subunits, beta and gamma chains, and the heterotrimerization of these chains leads to high-affinity binding for IL-2.Citation6 Several studies have shown that, in T-cell leukemia/lymphoma and Hodgkin lymphoma, CD25 is selectively overexpressed and chosen as the target for immunotherapy.Citation7,Citation8 Phase I/II clinical studies using anti-CD25 monoclonal antibody have already achieved encouraging results for such refractory lymphomas.Citation9,Citation10 Meanwhile, in mature DLBCL, previous studies demonstrated that CD25 is up-regulated in hairy cell leukemia (HCL) and B chronic lymphocytic leukemia (CLL),Citation11,Citation12 but it has not yet been examined in other subtypes of DLBCL including DLBCL. The soluble form of IL-2R (sIL-2R) is probably generated by the proteolytic cleavage of CD25 and, interestingly, several studies have shown that the levels of sIL-2R are frequently elevated and show a prognostic value in patients with DLBCL.Citation13–Citation15 The biological relevance between sIL-2R and CD25 remains unclear.
Hence, the aims of this study were to investigate whether CD25 is expressed in patients with DLBCL and associated with the prognosis. The results could shed light on new therapeutic approaches to DLBCL.
Materials and methods
Patients and histological examination
This study involved a cohort of 123 consecutive patients with newly diagnosed DLBCL who had been admitted to our hospital between 2005 and 2011. In contrast to DLBCL, 60 patients with newly diagnosed follicular lymphoma (FL) and 18 patients with reactive lymphadenopathy during the same period were involved. The study protocol was approved by the Bioethics Committee of Jichi Medical University.
All lymph nodes or related tissue biopsy samples from patients, who were diagnosed with DLBCL later, were obtained at the initial presentation. Collected samples were divided at least into two fractions: one for histological examination and the other for flow cytometric analysis. In histological examination, cells were fixed in formalin and stained with a solution containing hematoxylin-eosin and Wright-Giemsa. Immunostaining was performed using a panel of monoclonal antibodies including CD20 (L26; DakoCytomation, Glostrup, Denmark), CD10 (Dako), and CD3 (Dako). Histologic diagnosis was defined according to the 2008 World Health Organization (WHO) classification.Citation1 Patients diagnosed by cytology and flow cytometry (FCM) only and samples containing an insufficient number of B cells (CD19 or 20 < 70%) or a large number of T cells (CD3 > 90%) in a gated region by FCM were excluded.
Phenotyping
Single-color FCM was performed as routine practice.Citation16,Citation17 Samples were dissected and suspended in phosphate-buffered saline containing 1% bovine serum albumin to obtain single-cell suspensions. Then, cells were stained with a panel of fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies: CD1 (Dako, Carpinteria, CA, USA) CD2 (Dako), CD3 (Becton Dickinson Biosciences, San Jose, CA, USA), CD4 (Dako), CD5 (Becton), CD7 (Becton), CD8 (Dako), CD10 (Dako), CD19 (Dako), CD20 (Beckman Coulter, Fullerton, CA, USA), CD23 (Dako), CD11b (Beckman), CD11c (Beckman), CD14 (Beckman), CD13 (Beckman), CD33 (Beckman), CD25 (Becton), CD34 (Becton), CD117 (Beckman), CD56 (Becton), HLA-DR (Beckman), TCRαβ (Becton), TCRγδ (Becton), immunoglobulin (Biosource International, Camarillo, CA, USA or Dako), kappa light chain (Biosource or Dako), and lambda light chain (Biosource or Dako). For the negative controls, cells were stained with isotype-matched control antibodies (Becton). A lymphoid gate was set using forward and side scatter properties.
In another setting, cells were dually stained with one of the FITC-conjugated monoclonal antibodies and one of the PE-conjugated monoclonal antibodies.Citation16,Citation17 The combination of FITC-conjugated and PE-conjugated monoclonal antibodies used in this study was as follows: CD5/CD19, CD8/CD4, CD3/CD30 (Beckman), CD10/CD34, CD2/CD56, CD7/CD33, CD20/CD117, CD14/CD235a (Beckman), CD25/CD19, and CD25/CD20. For negative controls, cells were stained with FITC-conjugated mouse IgG1 (Becton) and PE-conjugated mouse IgG1 (Becton). A lymphoid gate was set using forward and side scatter properties.
Stained cells were analyzed using a flow cytometer (FACSCalibur; Becton). Flow cytometric data were evaluated by two independent FCM specialists. A minimum of 5000 events in the gated region were collected. The percentages of CD19+CD25+ cells and CD20+CD25+ cells were calculated as the ratios of CD19+CD25+ events and CD20+CD25+ events to 5000 events, respectively.
Statistical analysis
The χ2 test was used to compare the two groups for categorical data and Student's t-test for continuous data. The correlation between two variables was calculated by the Pearson correlation coefficient. Progression-free survival (PFS) was calculated from the date of diagnosis to disease progression, relapse, or death from any cause. Overall survival (OS) was calculated from the date of diagnosis until death from any cause. Patients still alive or lost to follow-up were censored at the date they were last known to be alive. PFS and OS were estimated by the Kaplan–Meier method, and were compared by means of the log-rank test. Multivariate analysis was performed with the Cox proportional hazard regression model. P values were two-sided and regarded as significant if less than 0.05. Data were analyzed using StatMate software version IV for Windows (ATMS, Tokyo, Japan).
Results
Expression of CD25 on lymphoma cells
To confirm CD25 expression on the lymphoma cells of DLBCL, two-color flow cytometric analysis was performed. As shown in A, CD25 expression was detected on both CD19+ and CD20+ lymphoma cells. The percentages (mean ± SD) of CD19+CD25+ and CD20+CD25+ cells were 63.7 ± 25.5% (n = 13) and 55 ± 28.1% (n = 14), respectively. We further studied whether CD25 expression was influenced by contaminated T-lymphocytes, especially regulatory T cells (Treg). The positivity (mean ± SD) of CD3, 4, and 8 in the gated regions was 14.1 ± 17.8, 29.6 ± 22.2, and 11.2 ± 12%, respectively. As shown in B, there was no correlation between CD25 and CD3, CD4, or CD8 expression (r = 0.014, P = 0.86; r = 0.112, P = 0.22; r = −0.028, P = 0.76, respectively).
Figure 1. CD25 expression in DLBCL. DLBCL cells are identified by forward and side scatter properties, and CD25 expression on the cells is shown using two-color flow cytometry (A). There is no relationship between CD25-positivity and one of CD3, CD4, and CD8-positivity (B) in the gated regions. r, correlation coefficient.
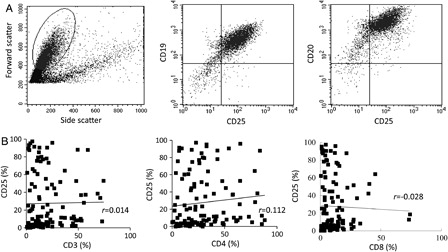
To identify CD25 expression in non-malignant lymph node lesions, CD25 expression was examined in patients with reactive lymphadenopathy; mean ± SD percent of CD25 was significantly lower in reactive lymph nodes (n = 16; 8.6 ± 4.3%) than in lymph nodes of DLBCL (n = 123; 27.8 ± 30.6%) (P < 0.001). Then, CD25 expression was surveyed in two major lymphomas, DLBCL and FL. The mean ± SD percent of CD25 was significantly higher in the samples of DLBCL (n = 123; 27.8 ± 30.6%) than in those of FL (n = 60; 12.7 ± 12.4%) (P < 0.001).
Survival analysis based on CD25 expression
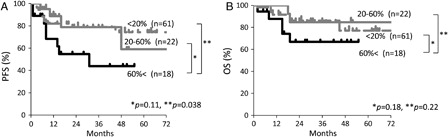
In this study, the 123 patients with DLBCL were treated with R-CHOP (n = 102), CHOP (n = 4), high-dose methotrexate-based therapy with or without rituximab (n = 5), and palliative therapy (n = 12). To evaluate the impact of CD25 expression on the prognosis, we first compared PFS and OS in the groups divided by cut-off values of 20, 40, 60, and 80% CD25-positivity, respectively. Kaplan–Meier curves showed poorer PFS and OS in each group with higher CD25-positivity than with equal or lower CD25-positivity. P values calculated by the log-rank test for PFS in each group were 0.2, 0.09, 0.046, and <0.01; for OS, 0.5, 0.2, 0.13, and <0.01, respectively. The number of patients with more than 80% CD25-positivity was only six, and such a small size was considered to be inadequate as the cut-off value. Therefore, a cut-off value of 60% CD25-positivity was established. Usually, reactivity is defined as positive when more than 20% of the cells are stained with a monoclonal antibody. Therefore, the other cut-off value of 20% CD25-positivity was introduced. Next, we compared the survival curves for R-CHOP-treated patients in accordance with the levels of this surface marker. Patients were divided into three groups: a group of patients with more than 60% CD25-positivity, that with 20–60% positivity, and that with less than 20% positivity. The Kaplan–Meier analysis revealed that both PFS and OS were inferior in patients in the first group than in the second or third group, despite being mostly non-significant (PFS, P = 0.11, 0.038; OS, P = 0.18, 0.22, respectively; ). Because the patients with more than 60% in CD25-positivity had a poor PFS, patients were divided into two groups: patients showing more than 60% CD25-positivity (CD25 high) and 60% or lower CD25-positivity (CD25 low).
Clinical features of CD25+ DLBCL patients
The characteristics of the patients with DLBCL are shown in . The mean CD25-positivity was 80.4% in the CD25-high group but 14.3% in the CD25-low group. Patients with CD25-high DLBCL showed an association with some poor clinical features such as B symptoms, chromosome abnormality, and an sIL-2R titer of more than 1000 U/ml, but they did not reach significance.
Table 1. Patient characteristics according to CD25 expression
Therapeutic response and prognosis of CD25+ DLBCL patients
The overall and complete response rates in patients treated with R-CHOP were 73, 68% in the CD25-high group and 90, 84% in the CD25-low group, respectively (P = 0.049, 0.01, respectively; ). Although the complete response rate was not significant, there was a more frequent relapse in the former than the latter (P = 0.042, ). Therefore, patients in the CD25-high group showed a significantly inferior PFS than those in the CD25-low group (P = 0.046; A). When multivariate analysis was performed for CD25-positivity and International Prognostic Index categories, CD25-positivity did not have a significant prognostic value for PFS (hazard risk, 1.7, 95%; confidence interval, 0.6–4.9; P = 0.31). Although the OS rate was also inferior in the high-CD25 group, the difference was not significant (P = 0.13, B).
Figure 3. Kaplan–Meier estimates of PFS (A) and OS (B) for patients with CD25-high and CD25-low DLBCL treated with R-CHOP. CD25-high and CD25-low indicate 60% or more CD25-positivity in the gated regions and less than 60% positivity in the regions, respectively.
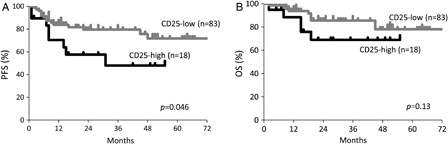
Table 2. Response to R-CHOP chemotherapy
In our cohort, the median sIL-2R was 2200 mg/dl, ranging from 300 to 84 900 in a total of 121 patients. As we predict the clinical outcome of patients with DLBCL treated with R-CHOP by the sIL-2R level, both PFS and OS were inferior in patients with levels of ≥2000 than <2000 (log-rank test, P < 0.001, <0.001, respectively). However, there was no significant correlation coefficient between levels of CD25 and sIL-2R (r = −0.036, P = 0.89).
Discussion
To the best of our knowledge, there have not been any studies focusing on CD25 expression regarding DLBCL. In this study, we were able to show CD25 expression in DLBCL by FCM, which was correlated with the shortened PFS and adverse prognosis.
Activated T cells, Treg, and NK cells express high levels of CD25, and the existence of high-affinity IL-2R is limited to these cell populations.Citation18,Citation19 First we considered the possibility that infiltrated T cells in biopsied samples would reflect CD25 expression. However, we did not find any correlation between the expression of CD25 and T-cell antigens (CD3, 4, 8). Second, to minimize the number of reactive infiltrated cells, we selected samples containing uniform B cells (CD19 or 20 ≥70%). Third, CD25-positivity in reactive lymph nodes was significantly lower than that in DLBCL. Finally, two-color FCM revealed that lymphoma cells of DLBCL actually expressed CD25. Consequently, these findings suggest that CD25 expression in the biopsy samples of DLBCL is mainly due to DLBCL cells.
Although they were not significant, aggressive clinical features such as B symptoms and chromosome abnormality were associated with CD25-high DLBCL. IL-2 was originally identified as a T-cell growth factor but IL-2/IL-2R signaling activates not only T cells but also normal and neoplastic B cells in growth and proliferation.Citation20 Therefore, CD25 expression could provide aggressiveness to DLBCL, and so further studies on the molecular biological mechanisms of CD25 expression in DLBCL are needed.
In this study, sIL-2R was significantly predictive of a poor outcome in patients with DLBCL, but we did not confirm an increase of sIL-2R in proportion to CD25 expression. Although the origin of sIL-2R has not been adequately investigated, this study indicated that high levels of sIL-2R are not associated with marked positivity of CD25 on lymphoma cells. Therefore, other mechanisms such as CD25 release from T or NK cells may be involved.Citation21
Recently, it was reported that CD25+ B cells were isolated from the peripheral blood of healthy individuals. They express higher levels of surface immunoglobulin but failed to secrete immunoglobulin,Citation22 and most of them belong to a subclass of memory B-cells.Citation23 Therefore, if CD25+ B cells are able to undergo malignant transformation during differentiation from memory B cells to plasma cells, this may represent a normal counterpart of not only CD25+ DLBCL.
In Epstein–Barr virus (EBV)-associated diseases such as Burkitt and Hodgkin lymphoma, EBV latent membrane protein-1 (LMP1) enhances CD25 expression on lymphoma cells through the NFkB pathway.Citation24 Regarding EBV infection, the 2008 WHO classification included the concept ‘EBV+ DLBCL of the elderly’ as a new provisional entity, and it presented the features with a higher age distribution and inferior prognosis.Citation1,Citation25 In this study, considering that 84% of the patients with CD25-high DLBCL were more than 60 years old, both EBV+ and CD25+ DLBCL may be overlapping entities, and this would be consistent in terms of the poor prognosis.
It was reported that CD25 expression was detected in CLL, HCL, and Hodgkin lymphoma. According to transformation from CLL/HCL to DLBCL and gray-zone lymphoma between DLBCL and Hodgkin, CD25 expression may have relevance to the pathophysiology and be used as a prognostic marker. Then, because CD25+ DLBCL patients showed a poor PFS following chemotherapy combined with anti-CD20 immunotherapy, a strategy targeting CD25 would have a clinical benefit for many subtypes including CD25+ DLBCL.
In conclusion, CD25+ DLBCL may constitute a distinct subgroup with aggressive clinical features and an inferior prognosis. As R-CHOP is less effective for these patients, an alternative approach such as R-CHOP plus anti-CD25 immunotherapy may be useful.
References
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040–5.
- Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91.
- Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9:105–16.
- Lowenthal JW, Zubler RH, Nabholz M, MacDonald HR. Similarities between interleukin-2 receptor number and affinity on activated B and T lymphocytes. Nature. 1985;315:669–72.
- Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8.
- Waldmann TA. Daclizumab (anti-Tac, Zenapax) in the treatment of leukemia/lymphoma. Oncogene. 2007;26:3699–703.
- Tesch H, Günther A, Abts H, Jücker M, Klein S, Krueger GR, et al. Expression of interleukin-2R alpha and interleukin-2R beta in Hodgkin's disease. Am J Pathol. 1993;142:1714–20.
- Dancey G, Violet J, Malaroda A, Green AJ, Sharma SK, Francis R, et al. A Phase I clinical trial of CHT-25 a 131I-labeled chimeric anti-CD25 antibody showing efficacy in patients with refractory lymphoma. Clin Cancer Res. 2009;15:7701–10.
- Ceesay MM, Matutes E, Taylor GP, Fields P, Cavenagh J, Simpson S, et al. Phase II study on combination therapy with CHOP-Zenapax or HTLV-I associated adult T-cell leukaemia/lymphoma (ATLL). Leuk Res. 2012;36:857–61.
- de Totero D, Francia di Celle P, Cignetti A, Foa R. The IL-2 receptor complex: expression and function on normal and leukemic B cells. Leukemia. 1995;9:1425–31.
- Tsilivakos V, Tsapis A, Kakolyris S, Iliakis P, Perraki M, Georgoulias V. Characterization of interleukin 2 receptors on B-cell chronic lymphocytic leukemia cells. Leukemia. 1994;8:1571–8.
- Goto N, Tsurumi H, Goto H, Shimomura YI, Kasahara S, Hara T, et al. Serum soluble interleukin-2 receptor (sIL-2R) level is associated with the outcome of patients with diffuse large B cell lymphoma treated with R-CHOP regimens. Ann Hematol. 2012;91:705–14.
- Ennishi D, Yokoyama M, Terui Y, Asai H, Sakajiri S, Mishima Y, et al. Soluble interleukin-2 receptor retains prognostic value in patients with diffuse large B-cell lymphoma receiving rituximab plus CHOP (RCHOP) therapy. Ann Oncol. 2009;20:526–33.
- Morito T, Fujihara M, Asaoku H, Tari A, Sato Y, Ichimura K, et al. Serum soluble interleukin-2 receptor level and immunophenotype are prognostic factors for patients with diffuse large B-cell lymphoma. Cancer Sci. 2009;100:1255–60.
- Oka S, Nagatsuka Y, Kikuchi J, Yokote T, Hirabayashi Y, Hanafusa T, et al. Preferential expression of phosphatidylglucoside along neutrophil differentiation pathway. Leuk Lymphoma. 2009;50:1190–7.
- Oka S, Muroi K, Matsuyama T, Sato K, Ueda M, Toshima M, et al. Correlation between flow cytometric identification of CD33-positive cells and morphological evaluation of myeloblasts in bone marrow of patients with acute myeloblastic leukemia. Hematology. 2009;14:133–8.
- Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–74.
- Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–39.
- Olejniczak K, Kasprzak A. Biological properties of interleukin 2 and its role in pathogenesis of selected diseases a review. Med Sci Monit. 2008;14:RA179–89.
- Lindqvist CA, Christiansson LH, Simonsson B, Enblad G, Olsson-Strömberg U, Loskog AS. T regulatory cells control T-cell proliferation partly by the release of soluble CD25 in patients with B-cell malignancies. Immunology. 2010;131:371–6.
- Brisslert M, Bokarewa M, Larsson P, Wing K, Collins LV, Tarkowski A. Phenotypic and functional characterization of human CD25+ B cells. Immunology. 2006;117:548–57.
- Amu S, Tarkowski A, Dörner T, Bokarewa M, Brisslert M, Scand J. The human immunomodulatory CD25+ B cell population belongs to the memory B cell pool. Immunology. 2007;66:77–86.
- Kube D, Vockerodt M, Weber O, Hell K, Wolf J, Haier B, et al. Expression of epstein-barr virus nuclear antigen 1 is associated with enhanced expression of CD25 in the Hodgkin cell line L428. J Virol. 1999;73:1630–6.
- Castillo JJ, Beltran BE, Miranda RN, Paydas S, Winer ES, Butera JN. Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly: what we know so far. Oncologist. 2011;16:87–96.