Abstract
The incidence of lymphoid malignancies has been increasing rapidly. Despite growing evidence for a relationship between serum 25-hydroxivitamin D [25(OH)D] concentrations and solid tumor risk, far less is known about the relationship between 25(OH)D and the risk of hematologic malignancy. This study aimed to assess the prognostic relevance of serum 25(OH)D concentrations in patients with B chronic lymphocytic leukemia (B-CLL) and non Hodgkin's lymphoma (NHL). The study was carried out on 195 newly diagnosed patients (75 B-CLL and 120 NHL) as well as 30 normal healthy controls. For all patients and normal controls serum 25(OH)D concentrations were assayed by enzyme-linked immunosorbent assay. Serum 25(OH)D levels were significantly lower in B-CLL and NHL patients as compared with normal controls (P = 0.00 for both). Also, there are significant associations between serum 25(OH)D levels and positive CD 38, positive ZAP 70 as well as Binet stages (χ2 = 16.071, 16.644, 21.134 respectively; P = 0.00 for all) in the B-CLL patient group. Moreover, there are significant associations between serum 25(OH)D status and international prognostic index (IPI), performance status (χ2 = 6.994, 9.212, P = 0.02, 0.01 respectively), but not with clinical stages (χ2 = 3.115, P = 0.539) in NHL. Multivariate analysis revealed that 25(OH)D insufficiency is an independent poor prognostic factor in both B-CLL and NHL patient groups. In conclusion, 25(OH)D insufficiency is an independent poor prognostic factor in patients with B-CLL and NHL. 25(OH)D might be a therapeutic target in lymphoid malignancies.
Introduction
The incidence of lymphoid malignancies has been increasing rapidly. Chronic lymphocytic leukemia shows a remarkable heterogeneity, with some patients having an almost normal lifespan, others surviving only several months after diagnosis despite intensive therapy.Citation1 Despite growing evidence for a relationship between 25(OH)D levels and solid tumor risk, far less is known about the relationship between 25(OH)D and the risk of hematologic malignancy.Citation2
Although the central role of 25(OH)D in maintaining serum calcium and skeletal homeostasis has long been appreciated, much recent work has demonstrated that 25(OH)D also has pleiotropic effects on cellular differentiation, proliferation, apoptosis, and angiogenesis.Citation3 25(OH)D insufficiency represents the first potentially modifiable prognostic marker in chronic lymphocytic leukemia (CLL).Citation4 These are some of the strongest findings between 25(OH)D and cancer outcome; although these findings are very provocative, they are preliminary and need to be validated in other studies. However, they raise the issue of whether 25(OH)D supplementation might aid in treatment for this malignancy, and thus should stimulate much more research.Citation5
The 25(OH)D receptor is highly expressed by CLL B-cells relative to normal B and T-cells and pharmacologic doses of 25(OH)D derivatives developed as therapeutic compounds have been shown to cause preferential in vitro cell killing in primary CLL cells through a p53-independent mechanism and induce caspase 3 and nine dependent apoptosis (e.g. mitochondrial pathway) of CLL B-cells in vitro.Citation6 Treatment of CLL B-cells with these 25(OH)D analogs was also associated with MAP kinase pathway activation and suppression of ERK activity. An effect of serum 25(OH)D levels on cellular processes of particular relevance to leukemogenesis. In addition, 25(OH)D has been shown to inhibit proliferation and induce differentiation of both lymphocytes and lymphoma cell lines on in vitro testing.Citation7 So, 25(OH)D was suggested as a prognostic marker, and might be a therapeutic target in lymphoid malignancies.Citation8
The aim of the current study was to assess serum 25(OH)D concentrations in correlation with B-CLL and NHL disease severity as well as patients’ outcome.
Patients and methods
The study was conducted on 195 newly diagnosed patients and 30 healthy controls. The patients groups were recruited from Mansoura Oncology Center. The study was approved by the research ethics committee and consents were obtained from all patients. The patients group was categorized as follows:
Group 1
Includes newly diagnosed 75 B-CLL patients (47♂; 28♀), median and age range were 57and 50–60 years, respectively. The diagnosis was based on the following:
| •. | All patients had an absolute lymphocyte count >5.0 × 109/l. | ||||
| •. | Leukemia cell immunophenotype and CLL score were done by flow cytometry to confirm each case (CD5, CD23, CD19, CD22, CD79b, FMC7, and SmIg). | ||||
Baseline clinical, laboratory were abstracted from medical records using a standardized protocol.
Group 2
Includes 120 NHL patients (68 ♂; 52♀), median and age range were 61 and 52–67 years, respectively. The diagnosis was based on the following:
| •. | LN biopsy: according to the World Health Organization classification, we grouped the subtypes for analysis into 68 cases with diffuse large B-cell (DLBCL), 16 cases with mantle cell lymphoma, 16 cases with follicular lymphoma (FL), 12 cases with lymphoplasmacytic lymphoma and 8 cases with Burkitt's lymphoma. | ||||
| •. | Cell immunophenotype was done by flow cytometry to confirm each case (CD 5, CD 23, CD 10, CD 19, CD 22, CD 34, TDT, FMC7, CD 103, and SmIg). | ||||
Baseline clinical, laboratory, and treatment data were abstracted from medical records using a standard protocol.
Patients were systematically followed for 5 years or until death. Disease progression and deaths were verified through medical record review.
Group 3
Includes 30 healthy control persons matched for age (median and range of age were 59 and 50–65 years, respectively) and sex (17 ♂; 13♀) with the patient groups
Specimen collection
| •. | One milliliter of EDTA blood samples were collected for complete blood counts from the investigated groups. | ||||
| •. | Two milliliters of EDTA blood were collected from CLL and NHL groups for immunophenotyping. Prognostic testing (ZAP-70 status, CD38 status) was performed using flow cytometry. | ||||
| •. | Two milliliters of serum samples were collected for 25(OH)D detection from three groups. | ||||
| •. | Blood samples of all patient groups (B-CLL and NHL) and healthy controls were collected at summer time from (May to September) at first time of diagnosis to avoid different seasonal variation of sun exposure. | ||||
Analysis of CD38 and ZAP 70 expression by flow cytometry
Analysis was done using the EPICS XL flow cytometer (Coulter Electronics, Hialeah, FL, USA). Samples were prepared using a three-color staining method. Isotype-matched negative control antibodies were used to separate positive from negative cells. Directly labeled monoclonal antibodies against the lymphoid antigens CD5-FITC, CD19-PerCP, and CD38- PE were used. B-CLL cells (CD5 + /CD19+) were gated. The degree of CD38 expression in this gated population was expressed as percentage positivity. Percentages of ZAP-70-positive CLL cells are determined with negative threshold cut-off values set using ZAP-70-stained normal B-cells, as well as isotype control-stained B-CLL cells. The cut-off point for ZAP-70-positive in B-CLL cells was ≥20% and for CD38-positive was ≥30%, respectively.Citation9
Serum 25(OH)D concentrations measurement
The DRG® 25(OH)D (EIA-3153) (DRG International, Mountainside, NJ, USA). is used for determination of serum 25(OH)D concentration in serum. This test kit is a competitive protein-binding assay for the measurement of 25(OH)D. Curve of the absorbance unit vs. concentration is generated using the results obtained from the calibrators. Concentrations of 25(OH)D present in the samples and controls were determined directly from this curve.
Statistical analysis
The statistical analysis of data were done by using Excel program and SPSS version 16 (statistical package for social science) (SPSS Version 16; SPSS Inc., Chicago, IL, USA). Qualitative data were described in the form of numbers and percentages. Quantitative data were described in the form of median and range. Statistical analysis was done by comparison between groups using chi-squared test and Fisher's exact tests. The probability of being by chance (P value) was calculated for all parameters (P is significant if ≤0.05 at confidence interval 95%). Lymphoma and CLL survival were defined as the time starting from the diagnosis to death due to disease, and overall survival (OS) was defined as the time from diagnosis to death due to any cause. Patients without an event or death were censored at time of last known follow-up. Kaplan–Meier curve was used to assess the association of 25(OH)D levels and patients outcome.
Results
In the present study, 25(OH)D insufficiency was defined as a serum concentration 11–20 ng/ml, 25(OH)D deficiency as a serum concentration ≤10 ng/ml and sufficient25(OH)D above 20 ng/ml according to the current threshold used by Mayo Medical Laboratories (http://www.mayomedicallaboratories.com).Citation10 According to this cut-off of 25(OH)D; B-CLL patients group was sub-classified into 12 cases had deficient 25(OH)D (16%), 42 cases had insufficient 25(OH)D (56%), and 21 cases with sufficient 25(OH)D level (28%). Furthermore, among NHL 16 cases had deficient (13%), 48 cases had insufficient (40%), and 56 cases with sufficient 25(OH)D level (47%). The deficient and insufficient 25(OH)D distribution in NHL histological subtypes were as follows: DLBCL (42 out of 68 cases; 62%); Burkitt's lymphoma (8 out of 8 cases, 100%), FL (10 out of 16 cases, 62%), mantle lymphoma (4 out of 16 cases, 25%); and lymphoplasmacytic lymphoma (0 out of 12 case).
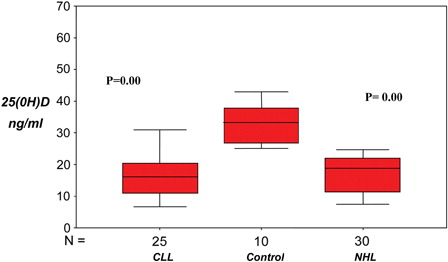
In the current study, significant reduction in serum 25(OH)D in B-CLL patients and NHL patients compared to control (P = 0.00). The median and range of serum 25(OH)D concentrations in B-CLL (16.1; 6.6–31.0 ng/ml), NHL (18.8; 7.5–24.7 ng/ml), and control groups (32.4; 25.0–40.8 ng/ml) ()
Figure 1. Median and range of 25(OH)D concentrations were in B-CLL (16.1; 6.6–31.0 ng/ml), in NHL (18.8; 7.5–24.7 ng/ml) and control groups (32.4; 25.0–40.8 ng/ml). There are significant reduction in serum 25(OH)D levels in B-CLL patients and NHL patients as compared with healthy controls (P = 0.00).
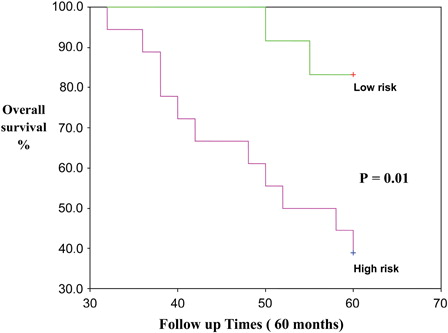
In the B-CLL group, there are significant associations between serum 25(OH)D levels and CD 38, ZAP 70, as well as Binet stages (χ2 = 16.071, 16.644, 21.134, respectively; P = 0.00 for all). The B-CLL patients group was classified into: high-risk group (25(OH)D deficiency and insufficiency with advanced Binet stage, positive CD 38, and positive ZAP 70) and low-risk group (25(OH) sufficiency, early Binet stage, negative CD 38, and negative ZAP 70) ().
Table 1. Association between 25(OH)D concentrations and prognostic markers inB-CLL patients
Furthermore, statistical studies in NHL, revealed significant association between serum 25(OH)D levels and performance status (χ2 = 6.994, P = 0.02) as well as IPI (χ2 = 9.212, P = 0.01), but not with clinical stages (χ2 = 3.115, P = 0.539). The NHL patients group was classified into high-risk group (25(OH)D deficiency and insufficiency with advanced clinical stage, advanced performance status, and high IPI) and low-risk group (25(OH) sufficiency, early clinical stage, early performance status, and low IPI) ().
Table 2. Association between 25(OH)D concentrations and prognostic markers of NHL patients
Univariate and multivariate Cox regression analysis was used to assess associations between survival and potential risk factors including Binet stage, ZAP-70-positive, CD 38-positive, serum 25(OH)D concentration in B CLL and clinical stages, performance status, and IPI serum 25(OH)D concentration in NHL. These studies revealed that the significant parameter was serum 25(OH)D concentration (P = 0.02) in BCLL and IPI (P = 0.08), serum 25(OH)D concentration (P = 0.03) in NHL patients. Multivariate analysis revealed that serum 25(OH)D levels are an independent prognostic factor associated with survival in B-CLL and NHL patients (Tables and ).
Table 3. Cox regression multivariate analysis of 25(OH)D and other prognostic factors in B-CLL
Table 4. Cox regression multivariate analysis of 25(OH)D and other prognostic factors in NHL
High-risk group B-CLL patients associated with significant inferior in the OS as compared with the low-risk group (P = 0.04). The estimated mean OS of B-CLL patient in low- and high-risk groups were 56.8 months (95% confidence interval, 52.3–61.4 months) and 48.7 months (95% confidence interval, 42.9–54.5 months), respectively. The death frequency in high-risk group patients was very high (36/51 cases were died). The death frequency in low-risk group patients was 6/24 cases ().
Figure 2. Kaplan–Meier curve for OS in high-risk B-CLL versus low-risk B-CLL patients subgroup. There are significant shortening in the OS in the high-risk group as compared with the low-risk group (P = 0.04).
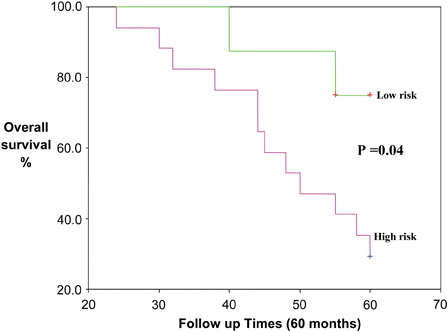
High-risk group NHL patients were associated with significant inferior OS as compared to low-risk group (P = 0.01). The estimated mean OS of NHL patient in low- and high-risk groups were 58.7 months (95% confidence interval, 57.07–60.4 months) and 50.8 months (95% confidence interval, 45.8–55.6 months), respectively. The death frequency in high-risk group patients (48/72 cases) was significantly higher as compared with that in low-risk group patients (28/48 cases) ().
Discussion
25(OH)D was involved in the regulation of vital cellular processes including differentiation, proliferation, apoptosis, and angiogenesis.Citation11,Citation12
In the current study, serum 25(OH)D concentrations were significantly lower in B-CLL and NHL patients when compared with controls. This is in agreement with the findings of Shanafelt et al.Citation13 and Drake et al.Citation14 Hickish et al.Citation15 and Ramagopalan et al.Citation16 mentioned that 25(OH)D is a steroid prohormone with anti-neoplastic properties. Moreover, Pepper et al.Citation17 reported similar finding and stated that pharmacological doses of 25(OH)D derivatives developed as therapeutic compounds have been shown to induce caspase 3- and 9-dependent apoptosis(e.g. mitochondrial pathway) of CLL B-cells in vitro. Furthermore, the relationship between lymphoid malignancy and 25(OH)D was suggested by Bai,Citation18 who stated that 25(OH)D increased inhibitors and decreased activators of cyclin–cyclin-dependent kinase complexes in addition to increasing levels of cyclin-dependent kinase inhibitors, Cip/Kip proteins P21 and P27. These proteins are known to keep the cell cycle in the G1/S phase, preventing calcium metabolism, and it was important not only for regulating cellular proliferation and differentiation but also for a wide variety of other biologic processes, including regulation of immune function, modulating vascular tone, and influencing rennin and insulin synthesis.
The relation between 25(OH)D insufficiency and NHL was emerged in many studies.Citation9,Citation19,Citation20 Erber et al.,Citation19 Egan et al.,Citation9 and Kricker et al.Citation20 suggested that 25(OH)D intake may be more protective against NHL; and found an association between 25(OH)D plus multivitamins to lower NHL risk in African Americans. They reported also that higher levels of sun exposure, which would be anticipated to increase 25(OH)D levels, were associated with a lower NHL risk. Armstrong and Kricker,Citation21 Freedman et al.,Citation22 Purdue et al.,Citation23 and Smedby et al.Citation24 hypothesize that 25(OH)D insufficiency increases non-Hodgkin's lymphoma risk through its antiproliferative and immunomodulatory effects. On the contrary, Hartge et al.,Citation25 and Soni et al.Citation26 provided limited support for an association between 25(OH)D status and lymphoma. They mentioned that the association of dietary 25(OH)D intake or serum 25(OH)D and lymphoma risk are largely weak or null. Bertrand et al.Citation27 observed no association between 25(OH)D measures and risk of NHL overall or by subtype. Chang et al.Citation28 support that the protective effect of routine residential UVR exposure against lymphomagenesis through mechanisms possibly independent of 25(OH)D. Kelly et al.Citation29 suggested that there is limited evidence of an association between 25(OH)D status and NHL risk may be due to methodological limitations, and further investigation of this potential association is warranted.
In the current study are significant associations between serum 25(OH)D concentrations and CLL prognostic factors (positive CD 38, positive ZAP70 as well as Binet stages). This is in agreement with the finding of Shanafelt et al.,Citation13 and Döhner et al.,Citation30 and they stated that 25(OH)D insufficiency is associated with poor prognostic factors. Also they suggested that the prognostic effect of 25(OH)D may be directly related to its impact on CLL and not simply a general host effect. Multivariate analysis revealed that 25(OH)D is an independent prognostic factor associated with survival in B-CLL and NHL patients. Shanafelt et al.Citation13 clearly demonstrate that 25(OH)D insufficiency is an independent risk factor in B-CLL.
The NHL histological subtypes associated most frequently with serum 25(OH)D deficiency as well as insufficiency are DLBCL and Burkitt's. This is in agreement with the finding of Drake et al.Citation14 On the one hand, Polesel et al.Citation31 found a 70% lower FL risk associated with high dietary 25(OH)D. Unfortunately, there is a lack of literature on the effect of dietary 25(OH)D on NHL subgroups. In contrast, Lim et al.Citation4 suggested that analyses by the common histological subtypes of NHL generally did not show an association with 25(OH)D levels. However, individuals with the highest concentrations of 25(OH)D were at lower risk of DLBCL, FL, and CLL/SLL. On the other hand, Chang et al.Citation32 found no association between NHL and 25(OH)D status considering all subtypes. Furthermore, evidence of an effect of 25(OH)D on lymphoma cells in particular has been observed by promotion of differentiation and anti-proliferative effects on a variety of lymphoma cells line in vitro, and by demonstrating tumor response to alfa calcidol in low-grade follicular, small-cleaved cell type, lymphoma.Citation33
In the present study, high-risk B-CLL and NHL patients with deficiency and insufficiency of 25(OH)D concentrations had significantly shorter OS as compared to those at low-risk with sufficient 25(OH)D concentrations (P = 0.05, P = 0.01, respectively). This is in agreement with Shanafelt et al.,Citation13 Drake et al.,Citation14 and Jelinek et al.,Citation34 who suggested that 25(OH)D insufficiency may be the first potentially modifiable risk factor associated with prognosis in newly diagnosed CLL; patients with insufficient levels of 25(OH)D progressed much faster and were about twice as likely to die as were patients with adequate levels of 25(OH)D. More recently, Shanafelt et al.Citation35 reported that 25(OH)D insufficiency is associated with lower survival in chronic lymphocytic leukemia patients. Nevertheless, the therapeutic efficiency of 25(OH)D in B-CLL has not been reported to date.
Drake et al.Citation14 found that deficient 25(OH)D was associated with inferior OS in aggressive NHL subtypes; and suggested that the prognostic effect of 25(OH)D might be directly related to its impact on the lymphoma and not simply a general host effect. They explained this finding on the basis that 25(OH)D is capable of modulating several critical cellular processes, including inhibition of carcinogenesis by induction of cellular differentiation, inhibition of proliferation and angiogenesis, and promotion of apoptosis. Normalization of 25(OH)D levels in NHL patients was linked to longer survival times, even after controlling for other factors associated with leukemia progression.
In conclusion: 25(OH)D insufficiency is poor prognostic index in patients with B-CLL and NHL. 25(OH)D, and might be a therapeutic target in lymphoid malignancies.
References
- Stephens JM. Chronic lymphocytic leukemia: economic burden and quality of life: literature review. Am J Ther. 2005;12:460–6.
- Giovannucci E. Vitamin D and cancer incidence in the Harvard cohorts. Ann Epidemiol. 2009;19:84–8.
- Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34.
- Lim U, Freedman DM, Hollis BW. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124:979–86.
- Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9.
- Lappe JM. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91.
- Arlet JB, Callens C, Hermine O, Darnige L, Macintyre E, Pouchot J, et al. Chronic lymphocytic leukaemia responsive to vitamin D administration. Br J Haematol. 2012;156:148–9.
- Toner CD, Davis CD, Milner JA. The vitamin D and cancer conundrum: aiming at a moving target. J Am Diet Assoc. 2010;110:1492–500.
- Egan KM, Sosman JA, Blot WJ. Sunlight and reduced risk of cancer: is the real story vitamin D? J Natl Cancer Inst. 2005;97:161–3.
- http://www.mayomedicallaboratories.com.
- Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1, 25-Dihydroxyvitamin D3 inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–20.
- Luong K, Nguyen LT. The beneficial role of vitamin D and its analogs in cancer treatment and prevention. Crit Rev Oncol Hematol. 2009;73:192–201.
- Shanafelt TD, Drake MT, Maurer MJ. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood. 2011;117:1492–8.
- Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, Micallef IN, et al. Vitamin D insufficiency and prognosis in non-Hodgkin's lymphoma. J Clin Oncol. 2010;28:4191–8.
- Hickish T, Cunningham D, Colston K. The effect of 1, 25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br J Cancer. 1993;68:668–72.
- Ramagopalan SV, Heger A, Berlanga AJ. ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease. Genome Res. 2010;20:1352–60.
- Pepper C, Thomas A, Hoy T, Milligan D, Bentley P, Fegan C. The vitamin D3 analog EB1089 induces apoptosis via a p53-independent mechanism involving p38 MAP kinase activation and suppression of ERK activity in B-cell chronic lymphocytic leukemia cells in vitro. Blood. 2003;101:2454–60.
- Bai M. Low expression of p27 protein combined with altered p53 and Rb/p16 expression status is associated with increased expression of cyclin A and cyclin B1 in diffuse large B-cell lymphomas. Mod Pathol. 2001;14:1105–13.
- Erber E, Maskarinec G, Lim U, Kolonel LN. Dietary Vitamin D and Risk of Non-Hodgkin Lymphoma: The Multiethnic Cohort. Br J Nutr. 2010;103:581–4.
- Kricker A, Armstrong BK, Hughes AM, Goumas C, Smedby KE, Zheng T, et al. Interlymph Consortium. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122:144–54.
- Armstrong BK, Kricker A. Sun exposure and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:396–400.
- Freedman DM, Kimlin MG, Hoffbeck RW, Alexander BH, Linet MS. Multiple indicators of ambient and personal ultraviolet radiation exposure and risk of non-Hodgkin lymphoma (United States). J Photochem Photobiol B. 2010;101:321–5.
- Purdue MP, Hartge P, Davis S, Cerhan JR, Colt JS, Cozen W, et al. Sun exposure, vitamin D receptor gene polymorphisms and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2007;18:989–99.
- Smedby KE, Hjalgrim H, Melbye M, Torrång A, Rostgaard K, Munksgaard L, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97:199–209.
- Hartge P, Lim U, Freedman DM, Colt JS, Cerhan JR, Cozen W, et al. Ultraviolet radiation, dietary vitamin D, and risk of non-Hodgkin lymphoma (United States). Cancer Causes Control. 2006;17:1045–52.
- Soni LK, Hou L, Gapstur SM, Weisenburger DD, Chiu BC. Sun exposure and non-Hodgkin lymphoma: A population-based, case-control study. Eur J Cancer. 2007;43:2388–95.
- Bertrand KA, Chang ET, Abel GA, Zhang SM, Spiegelman D, Qureshi AA, et al. Sunlight exposure, vitamin D, and risk of non-Hodgkin lymphoma in the Nurses' Health Study. Cancer Causes Control. 2011;22:1731–41.
- Chang ET, Canchola AJ, Cockburn M, Lu Y, Wang SS, Bernstein L, et al. Adulthood residential ultraviolet radiation, sun sensitivity, dietary vitamin D, and risk of lymphoid malignancies in the California Teachers Study. Blood. 2011;118:1591–9.
- Kelly JL, Friedberg JW, Calvi LM, van Wijngaarden E, Fisher SG. A case-control study of ultraviolet radiation exposure, vitamin D, and lymphoma risk in adults. Cancer Causes Control. 2010;21:1265–75.
- Döhner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–6.
- Polesel J, Talamini R, Montella M, Parpinel M, Dal Maso L, Crispo A, et al. Linoleic acid,vitamin D and other nutrient intakes in the risk of non-Hodgkin lymphoma: an Italian case-control study. Ann Oncol. 2006;17:713–8.
- Chang ET, Balter KM, Torrang A, Smedby KE, Melbye M, Sundstrom C, et al. Nutrient intake and risk of non-Hodgkin's lymphoma. Am J Epidemiol 2006;164:1222–32.
- Raina V, Cunningham D, Gilchrist N, Soukop M. Alfacalcidol is a nontoxic, effective treatment of follicular small-cleaved cell lymphoma. Br J Cancer. 1991;63:463–5.
- Jelinek DF, Tschumper RC, Geyer SM, Bone ND, Dewald GW, Hanson CA, et al. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukemia. Br J Haematol. 2001;115:854–61.
- Shanafelt TD, Witzig TE, Fink SR. Prospective evaluation of clonal evolution during long term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24:4634–41.