Abstract
Objective
Malaria is highly prevalent and endemic in tropical countries and carries a significant health burden. The detection of malaria by light microscopy of Giemsa-stained smears is the gold standard. There are many hematological abnormalities associated with malaria like anemia, thrombocytopenia, and leucopenia; however, none of these abnormalities are specific. The present study was undertaken to assess the utility of WBC scattergram in predicting the diagnosis of malaria.
Methods
In this study all cases diagnosed as Plasmodium vivax/Plasmodium falciparum infection on peripheral smear examination were included. Their complete blood counts and WBC scattergrams obtained from XT2000i were critically evaluated. Accordingly, sensitivity, specificity, positive predictive value (PPV), and negative predictive value of detection of malaria by abnormal WBC scattergram with and without abnormal blood counts were also calculated.
Results
A total of 2251 ethylendiaminetetraacetic acid samples were run on XT2000i hematology autoanalyzer. Out of these 148 cases of malaria were diagnosed on peripheral smear (128 P. vivax and 20 P. falciparum). While analyzing the WBC scattergrams, 233 cases including 124 (83.8%) malaria cases showed different abnormalities. Sensitivity and PPV for the diagnosis of malaria by abnormal WBC scattergram were 83.78 and 53.20%, respectively. This had increased to 98.60 and 57.25%, respectively, when cytopenias were included.
Discussion
Sysmex XT-2000i is capable of detecting specific abnormalities in WBC scattergram in patients with malaria. Therefore, the presence of an abnormal WBC scattergram with thrombocytopenia in a febrile patient helps the pathologist to clinch the diagnosis of malaria.
Keywords:
Introduction
Malaria is highly prevalent and endemic in tropical countries and carries a significant health burden. The detection of malaria by light microscopy of Giemsa-stained smears is the gold standard. However, it has its own limitations of being time consuming and requires well-trained staff. This has led to the development of several new techniques for detection of malaria like quantitative buffy coat examination using fluorescent dyes,Citation1 antigen-coated dipstick tests,Citation2 and polymerase chain reaction.Citation3 Although these tests are fairly sensitive in detecting the malaria parasite, they are expensive and not routinely available.
There are many hematological abnormalities associated with malaria like anemia, thrombocytopenia, and leucopenia; however, none of these abnormalities are specific. As complete blood count (CBC) is the baseline investigation to be ordered in patients with fever, any malaria detecting method added to it, could help in early detection of malaria and reduce its complications.Citation4
Many studies have been conducted all over the world using the automated blood cell counters like cell dyne hematology analyzer,Citation5 GEN S and LH750,Citation6 XE2100 and XS100iCitation7 in the evaluation of malarial detection. In the present study, the abnormalities of WBC scattergram by XT2000i were evaluated, which can give a clue to diagnosis of malaria and thereby alerting the pathologist to review the blood smears more carefully.
Materials and methods
In this study, all cases diagnosed as Plasmodium vivax/Plasmodium falciparum infection on peripheral smear examination from June 2011 to September 2011 were included. This is a descriptive study in which a retrospective analysis of all positive cases was done. Their complete blood counts and WBC scattergrams obtained from XT2000i were critically evaluated for any abnormal findings. The parasitemia was indirectly calculated on peripheral smear examination by counting the number of parasites in 25 fields. The total parasite count per microliter of blood was then calculated by:
The degree of parasitemia was then correlated with any abnormal findings on differential WBC counts or abnormal WBC scattergram patterns. Also during the same period all samples run on XT2000i were analyzed. The WBC scattergrams of non-malarial cases were also evaluated.
Accordingly, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of detection of malaria by abnormal WBC scattergram with and without abnormal blood counts were also calculated.
Results
A total of 2251 ethylendiaminetetraacetic acid samples were run on XT2000i hematology autoanalyzer from June to September 2011. Out of these, 148 cases of malaria were diagnosed on peripheral smear (128 P. vivax and 20 P. falciparum). Eighty-two were males and 66 were females, the age range was between 5 and 60 years with maximum cases falling between 14 and 25 years of age.
Bicytopenia was observed in 44.6% of cases followed by pancytopenia (21.60%), isolated thrombocytopenia (21.60%), and isolated anemia (9.40%; ).
Table 1. Hemogram findings in malaria cases
While analyzing the WBC scattergrams, 233 cases including 124 (83.80%) malaria cases showed different abnormalities (). Some cases showed more than one abnormality.
Table 2. Different abnormalities in WBC scattergram in malaria cases
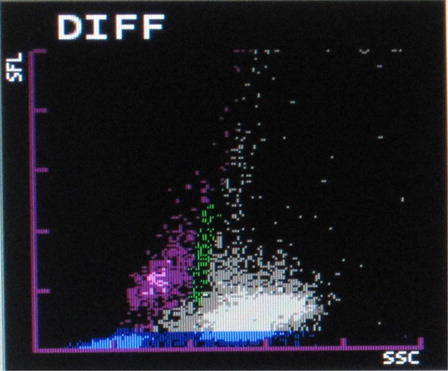
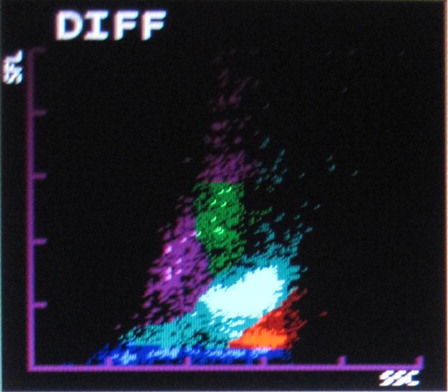
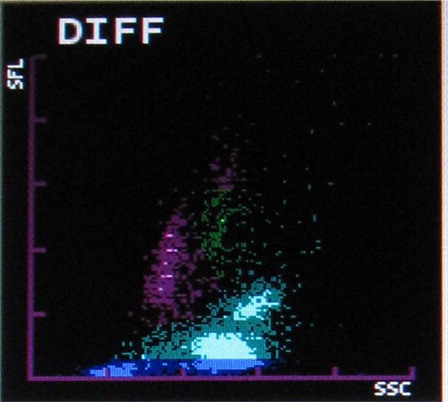
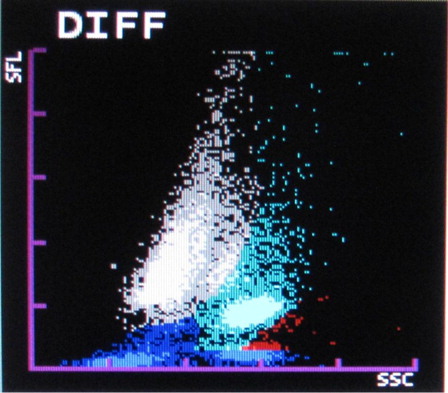
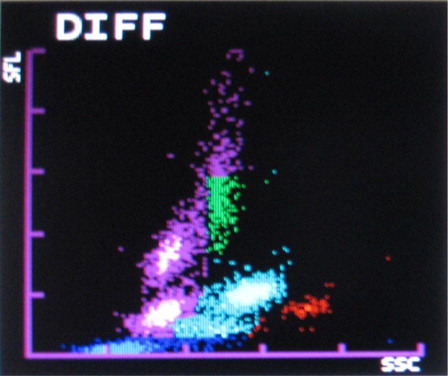
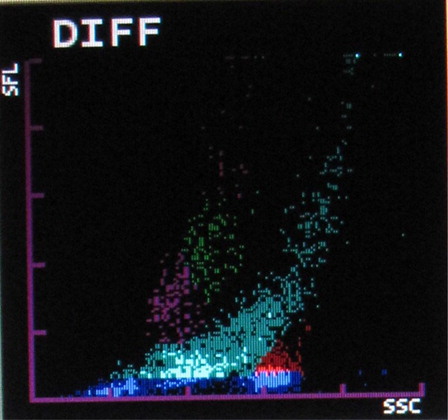
The most common abnormalities observed were graying of both eosinophil and neutrophil groups () (41.10%) followed by two eosinophil populations () (16.20%), overlapping of neutrophil and eosinophil groups () (16.20%), two neutrophil populations () (14.70%), graying of both lymphocyte and monocyte groups () (8.50%), graying of all leucocyte groups (2.30%), and two lymphocyte populations () (0.7%). It was also observed that as compared with only 4.90% abnormalities in P. vivax, 22.20% abnormalities of P. falciparum were showing graying of lymphocyte and monocyte groups. In the present study rightward shift of RBC ghost area in differential count (DIFF) () and WBC/basophil (BASO) scattergrams was also found in malaria cases (32.40 and 30.40%, respectively). In six cases (4%), spuriously high eosinophil counts were found in WBC scattergrams as compared with manual WBC differential count.
About 5.10% non-malaria cases like leukemias, thalassemias, septicemia, hemolytic diseases of newborn, and others also showed various abnormalities in WBC scattergrams as described above. Leukemia cases (31.20%) showed graying of all leucocyte groups in WBC scattergram. Diseases like thalassemias, septicemia and hemolytic diseases of newborn, having leucoerythroblastic blood picture (44%) showed graying of different leucocytes group, two neutrophil populations, and overlapping of neutrophil and eosinophil groups. Some cases (24.80%) of eosinophilia and smear with platelet aggregates also showed similar WBC scattergram abnormalities.
The peripheral blood films of all malaria cases were examined and total number of parasites was counted. However, no correlation was observed between degree of parasitemia and different patterns of WBC scattergram.
Sensitivity, specificity, PPV, and NPV were calculated on the basis of abnormal WBC scattergram findings with or without cytopenias in malarial positive cases (). The sensitivity and PPV for the diagnosis of malaria by abnormal WBC scattergram were 83.78 and 53.20%, respectively. This had increased to 98.60 and 57.25%, respectively, when cytopenias were included.
Table 3. Sensitivity, specificity, PPV, and NPV of abnormal scattergrams with or without cytopenias
Discussion
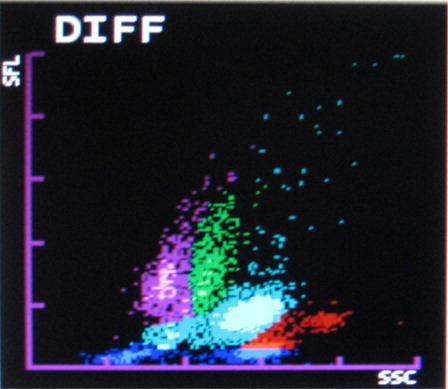
The Sysmex XT2000i hematology autoanalyzer measures different leucocytes by flow cytometry using a semiconductor laser beam and separates the cell by using the three signals, forward scatter, side scatter, and side fluorescence. The DIFF channel (differential count) uses surfactant to lyse red blood cells and platelets and a polymethine dye to bind to nucleic acid of WBC to give fluorescence signal intensity proportional to nucleic acid content. Also it uses an organic acid which specifically bind to eosinophil granules to be differentiated from neutrophils by high side scatter intensities. WBC/BASO channel uses forward and side scatter signals by using an acidic reagent which causes shrinkage of all cells except for basophils and WBC (bare nucleii). Thus the WBC and the basophil counts are derived from this channel.Citation8
The first automated hematology analyzer to detect malaria was Cell Dyn (CD) 3500 (Abott diagnostics, Santa, CA, USA) which detected hemozoin containing leucocytes especially monocytes abnormal depolarizing patterns during routine CBC analyses. This method was also beneficial in cases where there was no clinical suspicion of malaria.Citation5
Two case series from South Korea have shown the usefulness of pseudoeosinophilia and abnormalities in the DIFF scattergram in detecting malaria on SysmexXE-2100. Huh et al.Citation9 (2008) in their study of 144 malaria cases found 38.9% cases showing pseudoeosinophilia and 52.10% cases showing abnormalities in WBC scattergram like non-classified pot extending from neutrophils towards eosinophil area (22.90%), two eosinophil populations (21.50%), two neutrophil populations (27.80%) and overlapping of neutrophil and eosinophil populations (48.60%). This is caused when hemozoin containing particles interfere with the machine's WBC detection system. These abnormalities are due to the abnormal counting of hemozoin containing neutrophils as well as due to their detection as eosinophils near the neutrophil cluster. Yoo et al. in 2010 also studied the importance of pseudoeosinophilia and abnormal WBC scattergram in assessment of 413 malaria cases. They found 39% cases with pseudoeosinophilia and 15.70% cases with abnormal WBC scattergram like two neutrophil and two eosinophil populations.Citation10
In a Colombian study by Campuzano Zuluaga et al.Citation11 in 2010, the quantitative data, scatterplots and histograms were used to construct predictive models for each malaria species, by using binary logistic regression.
The present study showed 83.8% malaria cases having abnormal WBC scattergram. Most common abnormality was found to be graying of eosinophil and neutrophil populations (41.10%). Other common abnormalities were overlapping of eosinophil and neutrophil populations (16.20%) and two eosinophil populations (16.20%).
In the present study, rightward shift of RBC ghost in WBC/BASO and DIFF scattergrams were also very commonly found in malaria cases. This can be attributed to the presence of extracellular pigment and RBC lysis which are reflected in that area.Citation7
Although previous studies showed pseudoeosinophilia in approximately 39% of cases,Citation8,Citation9 the present study had only 4% cases showing spuriously high eosinophil count on scattergram as compared with smears, but it has shown little extra significance as time devoted for calculating it by microscopy will enable the observer to look for the parasite.Citation12
About six cases of P. falciparum showed graying of lymphocyte and monocyte groups, which might be due to the interference in their detection by ring forms of the parasite.Citation7
Huh et al.Citation8 (2008) showed in their study that XE-2100 had sensitivity of 69.40% and specificity of 100% while using pseudoeosinophilia and abnormal WBC scattergram in detection of malaria.
Yoo et al. (2010) reported sensitivity of 46.20% and specificity of 99.70% by using pseudoeosinophilia and abnormal WBC scattergram by Sysmex-2100. These parameters were low than above study because production of hemozoin depends on the number of parasites, severity of infection and host immunity factors.Citation9
The present study showed high sensitivity (83.78%) and specificity (94.82%) as compared with above studies while using abnormal WBC scattergram in detection of malaria. This sensitivity increased to 98.6% when abnormal scattergram findings were combined with cytopenias in the detection of malaria.
The present study showed that most cases had cytopenias in any of the blood cell lineage, common being anemias and thrombocytopenias. Several studies have been done which showed high incidence of thrombocytopenias and anemias in malaria cases.Citation13,Citation14 If the abnormal WBC scattergram findings are included with these hematological findings, the detection rate of malaria will definitely increase, as is shown in the present study.
Several new techniques have come up for the detection of malaria but requires special requisition by the treating clinician in patients suspected of malaria infection; however, the abnormal WBC scattergram findings may alert the pathologist to look for malaria in subclinical cases also.Citation15 As, CBC is the most frequently requested investigation in patients with fever, if the laboratory staff is aware of these WBC scattergram abnormalities, they will be more careful to look for the presence of parasite in blood smears.
References
- Levine RA, Wardlaw SC, Patton CL. Detection of hematoparasites using quantitative buffy coat analysis tubes. Parasitol Today. 1989;5:132–4.
- World Health Organization. WHO information consultation on recent advances in diagnostic techniques and vaccines for marlaria: a rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. Bull WHO. 1996;74:47–54.
- Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the 4 human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–92.
- Shapiro MF, Greenfield S. The complete blood count and leukocyte differential count. An approach to their rational application. Ann Intern Med. 1987;106:65–74.
- Mendelow BV, Lyons C, Nhlangothi P, Tana M, Munster M, Wypkema E, et al. Automated malaria detection by depolarization of laser light. Br J Haematol. 1999;104:499–503.
- Fourcade C, Casbas MJ, Belaouni H, Gonzalez JJ, Garcia PJ, Pepio MA. Automated detection of malaria by means of the haematology analyser Coulter GEN.S. Clin Lab Haematol. 2004;26:367–72.
- Yan F, Dai Y, Zhang Z, Wan H. The correlation of abnormal information in Sysmex hematology analyzers XE-2100 and XS-1000i with diagnosis of Plasmodium infection. Sysmex J Int. 2008;18:50–3.
- Fluorescence differentiation supports malaria diagnostics May 2006. Sysmex.
- Huh HJ, Oh GY, Huh JW, Chae SL. Malaria detection with the Sysmex XE-2100 hematology analyzer using pseudoeosinophilia and abnormal WBC scattergram. Ann Hematol. 2008;87:755–9.
- Yoo JH, Song J, Lee KA, Sun YK, Kim YA, Park TS, et al. Automated detection of malaria-associated pseudoeosinophilia and abnormal WBC scattergram by the Sysmex XE-2100 hematology analyzer: a clinical study with 1801 patients and real-time quantitative PCR analysis in vivax malaria-endemic area. Am J Trop Med Hyg. 2010;82:412–4.
- Zuluaga GC, Sanchez GA, Gallo GEE, Zuluaga LMV, Orrego AMR, Vidal AP, et al. Design of malaria diagnostic criteria for the Sysmex XE-2100 hematology analyzer. Am J Trop Med Hyg. 2010;82:402–11.
- Zuluaga GC, Hänscheid T, Grobusch MP. Automated haematology analysis to diagnose Malaria. Malar J. 2010;9:346.
- Chai JY. Re-emerging Plasmodium vivax malaria in the Republic of Korea. Korean J Parasitol. 1999;37:129–43.
- Fialon P, Macaigne F, Becker M, Boisseau MR, Cazenave J, Ripert C, et al. Hematological features in imported malaria. Value for the diagnosis of forms with low parasitemia. Pathol Biol (Paris). 1991;39:122–5.
- Hanschied T, Pinto BG, Pereira I, Cristino JM, Valadas E. Avoiding misdiagnosis of malaria: a novel automated method allows specific diagnosis, even in the absence of clinical suspicion. Emerg Infect Dis. 1999;5:836–8.