Abstract
Background
We investigated the clinical course and mortality of acute respiratory distress syndrome (ARDS) in patients with hematological malignancies.
Methods
Sixty-eight patients with hematological malignancies and ARDS admitted to medical intensive care unit (ICU) of a university hospital were analyzed semi-prospectively in the study.
Results
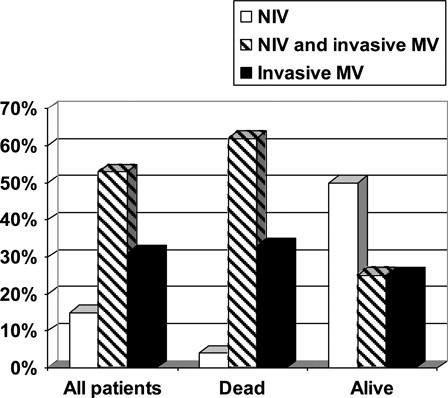
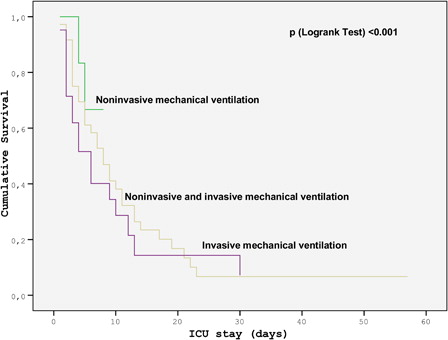
The most common etiology of ARDS was pneumonia. The ratio of partial pressure of oxygen in arterial blood to fractional concentration of inspired oxygen (PO2/FiO2) was 104 (74–165). Ten patients (15%) received non-invasive mechanical ventilation (NIV), 21 (31%) received invasive mechanical ventilation (MV), and 36 (53%) received both NIV and invasive MV. ICU mortality was 77% in the cohort. None of the variables with relevance to the underlying hematological disease was associated with mortality. The presence of two or more organ failures was the only independent risk factor for mortality (P = 0.045), whereas NIV was associated with low mortality (P = 0.001). The Kaplan–Meier curve of mortality, with respect to the type of MV support, demonstrated that NIV was associated with the lowest mortality (P < 0.001).
Conclusion
The mortality of ARDS in critically ill patients with hematological malignancies is quite high. The presence of multi-organ failure is independently associated with high mortality whereas the use of NIV is independently associated with low mortality.
Introduction
Acute respiratory distress syndrome (ARDS), characterized by diffuse alveolar damage associated with increased permeability of the alveolar–capillary membrane, is the most severe form of acute hypoxemic respiratory failure. In spite of recent improvements in the management of ARDS, it still has considerable mortality of up to 60%.Citation1 Advances in anticancer treatments and improvements in intensive care are shown to ameliorate the outcome of critically ill patients with hematological malignancies according to recent reports.Citation2 Promising results gave rise to an increase in the number of admissions to intensive care units (ICUs) with time. These patients are at risk for ICU admission not only due to agressive treatment strategies such as high-dose chemotherapy and hematopoietic stem cell transplantation (HCT), but to the immunosuppresion due to the underlying disease as well.Citation3–Citation5 Prompt diagnosis of the condition and transfer of these patients to ICUs are crucial.Citation6 Requirement of mechanical ventilation,Citation5 use of vasopresser agents,Citation5,Citation6 allogeneic HCT,Citation4,Citation6 presence of neutropenia,Citation5 hemodialysis,Citation5 sepsis,Citation4,Citation6 and multi-organ failureCitation4,Citation7 are the previously defined prognostic factors for mortality. Acute hypoxemic respiratory failure, which is seen almost in two-thirds of the patients with hematological malignancies requiring intensive care, is the leading cause of admission to ICUs.Citation8 Clinical course and prognosis varies considerably in the patients admitted with respiratory failure who meet the criteria for ARDS. Several previous reports refer to the increased mortality associated with acute hypoxemic respiratory failure in critically ill patients with hematological malignancies,Citation4,Citation9,Citation10 whereas there are limited specific data regarding the clinical characteristics and outcome of ARDS in patients with hematological malignancies. Our aim is to present the clinical course and outcome of ARDS in critically ill patients with hematological malignancies.
Materials and methods
This study was conducted in a nine-bed medical ICU located in Gazi University Hospital, Ankara, Turkey. The Institutional Ethical Committee of Gazi University Hospital approved the study. Written informed consent was obtained from all patients involved in the study. The hospital is a 1000-bed university hospital that has an eight-bed HCT unit where approximately 80 stem cell transplants are performed annually.
Study design and patient selection
Patients with hematological malignancies admitted to ICUs and met the criteria of ARDS were included in the study. ARDS was defined by the 1994 American-European Consensus Conference criteria.Citation11 The study population included both prospective and retrospective cohorts (28 prospectively and 40 retrospectively). The prospective cohort consisted of patients with hematological malignancies admitted to ICUs and met the criteria of ARDS between September 2009 and September 2010. Of the 250 patients admitted to the ICU during this period, 41 patients had hematological malignancies and 28 of these patients met the criteria of ARDS. For the retrospective cohort, we used the ICU database routinely collected prospectively during ICU admissions between April 2007 and September 2009. The data were mined for a cohort with hematological malignancy and the diagnosis of ARDS. A total of 158 patients with hematological malignancies were identified through the ICU database, 40 of these patients had the admission diagnosis of ARDS. So, totally 68 patients were included in the study. The partly prospective and partly retrospective data gave an opportunity to analyze patients admitted to ICUs during 3 years period. The prospective data provided more valuable information whereas retrospective data enabled analyzing various ICU practices with changing literature.
The epidemiologic and clinical data of patients were obtained from the prospectively created ICU database in the retrospective cohort, whereas they were collected prospectively by an internal medicine resident in the prospective group. Data included: demographics, Admission Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Glasgow Coma Scale (GCS), The Sequential Organ Failure Assessment (SOFA) score, and organ failures on admission (SOFA score of ≥3 for each organ in SOFA score).Citation12 The presence of acute kidney injury (acute kidney risk, injury or failure as defined by RIFLE classification), the original admission location of the patients, type and status (presence or absence of complete remission in the last hematological assessment) of the underlying hematological malignancy, history of HCT, the interval between transplantation to ICU admission, presence of graft-versus-host disease (GVHD), presence of neutropenia (an absolute neutrophil count of <500 cells/μl), application of chemotherapy in the ICU or the last 6 months prior to ICU admission, etiology of ARDS, presence of specific infections due to fungus, cytomegalovirus (CMV) or Pneumocystis jiroveci, cardiorespiratory and laboratory parameters on admission, type of respiratory support (non-invasive mechanical ventilation (NIV) or invasive mechanical ventilation (MV)), development of infection and severe sepsis during ICU stay, length of ICU stay and in the hospital, and finally the outcome of the patients were also recorded.
NIV was generally preferred when appropriate and the threshold of transition from NIV to invasive MV was kept high. Intolerance to NIV, failure to maintain a partial pressure of oxygen in arterial blood/fractional concentration of inspired oxygen ratio (PaO2/FiO2) of >85 during use of NIV, the development of conditions requiring intubation to protect the airways (seizures or severe encephalopathy with a score of 8 or less on the GCS), severe hemodynamic instability with worsening of metabolic acidosis, defined as a requirement of noradrenalin at doses of >5 µg/minute or dopamine at doses of >5 µg/kg/minute accompanied by a pH of 7.30 or less, presence of cardiac instability, defined as an evidence on electrocardiography of ischemia or clinically significant ventricular arrhythmias, and presence of copious tracheal secretions were the criteria for intubation.Citation13 NIV was delivered with a face mask and the ventilator (Servo-i Universal, MAQUET GmbH & Co. KG, Rastatt, Germany) set in the pressure-support or pressure-control mode. During NIV, the level of pressure support was progressively increased and adjusted for each patient to obtain an expired tidal volume of 7–10 ml/kg of body weight and respiratory rate fewer than 25 breaths per minute. Positive end expiratory pressure (PEEP) was routinely set at 4 cm of water and repeatedly increased by 1 cm of water up to a level of 10 cm of water, until the FiO2 requirement was 65% or less. The FiO2 was adjusted to maintain the arterial oxygen saturation (SpO2) above 90%. In invasive MV, assist control (pressure or volume targeted) mode was used with a low tidal volume and high PEEP ventilation and plato pressure was always kept <30 cm of water. In patients with refractory hypoxia, i.e. SpO2 < 90%, despite a FiO2 of >60%, prone position was implemented in the absence of a contraindication.Citation14,Citation15 All patients were preferentially fed by enteral nutrition with an enteral formulation enriched with Omega-3 fatty acids unless there was a contraindication. All the decisions regarding the patients were made with the consensus of the intensivist, hematologist, and infectious diseases consultants in the daily rounds.
Generally non-invasive, rarely invasive (bronchoalveolar lavage) diagnostic interventions were applied to uncover the etiology of ARDS. Etiological classification was made as pneumonia, sepsis, and unknown etiology. Patients who developed sepsis in the setting of known pneumonia were listed under the diagnosis of pneumonia.Citation16 The diagnosis of nosocomial infection was made according to the criteria of Centers for Diseases Control and Prevention.Citation17 The diagnosis of specific infections due to fungus, CMV, and Pneumocystis jiroveci was based on the presence of typical infiltrates on chest CT, sputum analysis, endotracheal aspirate or bronchoalveolar lavage fluid examination, serological and molecular markers. The definition and treatment of sepsis was made according to the Surviving Sepsis Campaign recommendations.Citation18
Statistical analysis
SPSS, version 15, software (SPSS, Chicago, IL, USA) was used for statistical analysis. Descriptive statistics were performed for demonstrating the general characteristics of patients and clinical features of ARDS. Comparisons were performed by χ2 or Fisher's exact test where appropriate for categorical variables and by Mann–Whitney U for continuous variables. Multivariate analysis was performed to find out the independent factors contributing the mortality in critically ill ARDS patients with hematological malignancies. Results were presented as number (percentage) for categorical variables and median (interquartile range) for continuous variables. The Kaplan–Meier curve was performed for the type of mechanical ventilation support and log-rank test was used for comparisons. P < 0.05 was considered as statistically significant.
Results
Characteristics of the study population
The characteristics of the patients are shown in . Sixty-nine percent of the patients were male with a median age of 45 (28–57), median APACHE II, GCS, and SOFA scores were 24 (19–32), 15 (11–15) and 10 (8–13), respectively. Leukemia (60%) was the most common hematological malignancy in the cohort. Thirty-two (47%) patients had undergone HCT prior to ICU admission, 91% of whom were allogenic. The median time interval between transplantation to ICU admission was 116 (24–300) days. The patients who succumbed and survived were similar with respect to age, gender, APACHE II score, GCS, SOFA score, organ failure, the original admission place, underlying hematological malignancy, presence of HCT, interval between transplantation to ICU admission, presence of GVHD, chemotherapy on and before the ICU admission, nosocomial infection during stay in the ICU, severe sepsis developed in the ICU, length of stay in the ICU or hospital. However, the presence of two or more organ failure, presence of acute kidney failure, and presence of neutropenia were higher in the group who succumbed to their disease (P = 0.011, 0.018 and 0.023) (). All the neutropenic patients had severe neutropenia (absolute neutrophil count <500 /μl). The duration of neutropenia could not be calculated due to inadequte data. Median lymphocyte count was 80 (5–674) in patients with leucopenia. Granulocyte-stimulating factor was used in 17 (45%) of 38 neutropenic patients, all of whom died.
Table 1. General characteristics of patients
The clinical characteristics of ARDS are shown in . The most common etiology of ARDS was pneumonia. The median PO2/FiO2 ratio was 104 (74–165). Non-invasive mechanical ventilation was used in 46 of 68 (68%) patients, and 36 of these patients required invasive MV subsequently and referred as NIV failure. Ten (15%) patients received only NIV and 22 (32%) patients received only invasive MV (, ).
Table 2. Clinical characteristics of acute respiratory distress syndrome
The determinants of NIV success were less severe illness and less severe acute respiratory failure indicated by lower APACHE II score and a higher PaO2/FiO2 ratio was found in patients who succeeded NIV (P = 0.002 and P = 0.025 respectively) (). The statistically significant variables for NIV success found in the univariate analysis are shown in .
Table 3. Univariate analysis for non-invasive mechanical ventilation success
Mortality
Fifty-two (77%) ARDS patients with hematological malignancies died during the ICU stay. Additional two patients died in the hospital after their discharge from the ICU (). Death occurred in the first 48 hours of ICU admission in 10 of the 52 patients who died in the ICU. Seven (10%) of the patients were still alive at 6 months follow-up after their discharge from the hospital.
Tables and show the comparison of patients who survived and succumbed during their ICU stay. Except neutropenia, the baseline demographics and medical history with respect to hematological malignancy were similar in the two groups. Several factors were found to be associated with ICU mortality (Tables and ). However, the variables found to be associated with mortality including the presence of two or more organ failures in addition to respiratory failure, use of NIV and presence of NIV failure were further analyzed in multivariate analysis. The presence of two or more organ failure in addition to respiratory failure was the only factor associated with mortality (odds ratio 6.61, confidence interval 1.04 − 41.93, P = 0.045) in multivariate analysis. NIV, on the other hand, was found to be associated with lower mortality (odds ratio 0.04, confidence interval 0.01 − 0.26, P = 0.001). The Kaplan–Meier curve showing mortality with respect to the type of MV support is shown in . Mortality varied with respect to the type of MV support, and NIV only was found to be associated with the lowest mortality (P < 0.001) ().
Discussion
Despite the recent improvements in the management of ARDS, it continues to be a major cause of morbidity and mortality in the ICU.Citation1 In this retrospective and prospective cohort study, we studied 68 ARDS patients with hematological malignancies. The mortality rate was 77% in the cohort. We have demonstrated that the variables of the underlying hematological malignancy did not affect the mortality after development of ARDS. Multi-organ failure was the only independent predictor of high mortality, whereas NIV was associated with lower mortality.
Acute respiratory failure is frequent and is a severe complication in patients with hematological malignancies and it is the leading reason of ICU admission in these patients.Citation8 ARDS is the most severe form of acute respiratory failure with a high mortality even in non-cancer patients.Citation1 The clinical characteristics and outcome of acute respiratory failure in patients with hematological malignancies has been studied in several previous reports.Citation9,Citation10 The characteristics and mortality of ARDS in general ICU population has also been studied extensively.Citation1 The present study is the first study specifically designed to investigate the characteristics and outcome of ARDS in patients with hematological malignancies to the best of our knowledge. Although Azoulay et al.Citation19 reported the results of 20 acute monocytic leukemia patients who were treated with dexamethasone in ICU due to respiratory symptoms probably due to hyperleucocytosis, the severity of lung injury was less severe than ARDS in majority of their cases. The ARDS mortality in critically ill patients varies between 27 and 64% depending on the specific patient group, underlying etiology, and the center or the country where the study is conducted.Citation1,Citation20,Citation21 Mortality could be as low as 27% in trauma patients, whereas it could be as high as 60% in the geriatric population.Citation20,Citation21 Thus, the ICU patient group should be specified to compare the outcome in patients with ARDS. There are only two studies addressing the risk factors for ARDS during recovery from neutropenia, in patients with hematological malignancies.Citation22,Citation23 These two studies by Azaoulay et al. and Ree et al. had a relatively low sample size with 21 and 38 ARDS patients, respectively, compared to our study. Their mortality was 62 and 87%, respectively, which compares favorably with our results and is in the upper end of the spectrum of mortality in ARDS.Citation22,Citation23
Hilbert et al.Citation9 reported a survival benefit of NIV in immunocompromised patients with acute respiratory failure and pneumonia in a randomized study in 2001. Thereafter NIV has become a preferred type of MV support in the clinical practice of these patients. However, later studies raised the concerns regarding the use of NIV in these patients due to the increased mortality rate with NIV failure, which was seen almost in half of the patients.Citation10,Citation24 In these studies, ARDS was defined as one of the predictors for NIV failure.Citation10,Citation24 Adda et al.Citation10 reported that NIV failure was experienced in half of the critically ill hematological patients with acute respiratory failure and was defined as an independent risk factor for mortality. They also reported that ARDS was the most important risk factor for NIV failure, increasing failure nearly 80 times.Citation10 In contrast, Depuydt et al.Citation25 demonstrated that mortality was not affected by the type of respiratory support but by the severity of the respiratory failure in 137 hematological patients most of whom had ARDS. The predictors of NIV failure in the management of NIV therapy in immunocompromised patients with acute respiratory failure came into prominence after these studies. Soares et al.Citation26 described an algorithm for critically ill patients with malignancies and acute respiratory failure in a recent article where ARDS was determined as one of the conditions invasive MV should be considered preferentially. Although the existing data do not encourage to use NIV in patients with hematological malignancies in ARDS, we used NIV in 46 out of 68 patients. We found that NIV in ARDS patients with hematological malignancies was independently associated with a relatively low mortality. We thus believe that NIV failure indicates more severe lung injury and is the consequence rather than being an indicator of more severe disease and NIV should be challenged when appropriate. Although we could not find any independent relationship between NIV failure and high mortality rate in ARDS in contrast to studies mentioned above,Citation10,Citation24 it might be an interesting research topic to determine the predictors of response to NIV in further studies. Thus, it would be possible to implement risk adoptive strategies by applying NIV to less severe cases and to proceed with invasive MV in high-risk patients to obviate a delay in treatment. Kaplan–Meier curve of mortality, with respect to the type of MV support, also indicated that NIV was associated with the lowest mortality. The success rate of NIV was 1 out of 32 ARDS patients (3%) in the study of Adda et al., which is far less than ours, 10 out of 46 patients (22%). However, most of our patients (n = 36) failed NIV and required further support with invasive MV. The patients who succeeded with NIV only had lower APACHE II and SOFA scores and higher PaO2/FiO2 on admission, which suggests that NIV success is associated with less severe pulmonary disease. Furthermore, lower incidence of nosocomial infection and severe sepsis during ICU stay was also associated with NIV success, which suggests a less complicated ICU course in patients who overcame ARDS with NIV only. Our results indicate that, patients with less severe critical illness with low APACHE II score and patients with less severe acute lung injury might benefit more from NIV. This raises the question whether response to NIV determines a group of patients with a relatively good prognosis or patients who already are low risk respond to NIV and warrants validation in future prospective trials with a larger sample size.
Our study has some limitations such as being a single center study with a relatively small sample size and its semi-prospective nature. Further prospective multi-center trials are required to support our findings and to investigate the predictors of response to NIV in patients with hematological malignancies and ARDS to implement risk-adapted strategies.
Non-invasive diagnostic strategies in the differential diagnosis might be another limitation of the current study. Although non-invasive strategies were not found to be inferior to the invasive techniques in some previous studies,Citation3 we believe that detailed diagnostic studies including invasive diagnostic procedures should be carried out to uncover epidemiological data related to ARDS in hematological malignancies. Despite some limitations the current study is the first and the largest study investigating clinical course of ARDS, specifically in critically ill patients with hematological malignancies.
Conclusions
In conclusion, the mortality of ARDS is higher than the general ICU population in patients with hematological malignancies. Our results suggest that the underlying hematological malignancy does not seem to have an impact on the outcome and the presence of multi-organ failure is the only independent predictor of high mortality. The 23% discharge rate should not be underestimated in such a high-risk group. Contrary to the present literature which disencourages NIV in ARDS patients with hematologic malignancies, our data suggest that at least a subset of patients might benefit from NIV, and NIV might in fact decrease infectious complications and perhaps mortality by avoiding intubation. Further prospective trials are required to find out the predictors of NIV success for managing these patients with NIV to improve the outcome.
Acknowledgements
The authors thank internal medicine residents for their contribution to the recording of the data. The authors also thank the nursing staff of the medical ICU of Gazi University for their excellent patient care.
References
- Ferguson ND, Frutos-Vivar F, Esteban A, Anzueto A, Alía I, Brower RG, et al. Mechanical Ventilation International Study Group. Airway pressures, tidal volumes, and mortality in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:21–30.
- Vandijck DM, Benoit DD, Depuydt PO, Offner FC, Blot SI, Van Tilborgh AK, et al. Impact of recent intravenous chemotherapy on outcome in severe sepsis and septic shock patients with hematological malignancies. Intensive Care Med. 2008;34:847–55.
- Azoulay E, Mokart D, Lambert J, Lemiale V, Rabbat A, Kouatchet A, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med. 2010;182:1038–46.
- Afessa B, Tefferi A, Dunn WF, Litzow MR, Peters SG. Intensive care unit support and Acute Physiology and Chronic Health Evaluation III performance in hematopoietic stem cell transplant recipients. Crit Care Med. 2003;31:1715–21.
- Huynh TN, Weigt SS, Belperio JA, Territo M, Keane MP. Outcome and prognostic indicators of patients with hematopoietic stem cell transplants admitted to the intensive care unit. J Transplant. 2009;2009:917294. Epub 2009 Sept 15.
- Hayani O, Al-Beihany A, Zarychanski R, Chou A, Kharaba A, Baxter A, et al. Impact of critical care outreach on hematopoietic stem cell transplant recipients: a cohort study. Bone Marrow Transplant. 2011;46:1138–44.
- Vandijck DM, Depuydt PO, Offner FC, Nollet J, Peleman RA, Steel E, et al. Impact of organ dysfunction on mortality in ICU patients with hematologic malignancies. Intensive Care Med. 2010;36:1744–50.
- Türkoğlu M, Mirza E, Tunçcan OG, Erdem GU, Dizbay M, Yağcı M, et al. Acinetobacter baumannii infection in patients with hematological malignancies in intensive care unit: risk factors and impact on mortality. J Crit Care. 2011;26:460–7.
- Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–7.
- Adda M, Coquet I, Darmon M, Thiery G, Schlemmer B, Azoulay E. Predictors of noninvasive ventilation failure in patients with hematological malignancy and acute respiratory failure. Crit Care Med. 2008;36:2766–72.
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24.
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
- Squadrone V, Massaia M, Bruno B, Marmont F, Falda M, Bagna C, et al. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med. 2010;36:1666–74.
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8
- Raoof S, Goulet K, Esan A, Hess DR, Sessler CN. Severe hypoxemic respiratory failure: part 2–nonventilatory strategies. Chest. 2010;137:1437–48.
- Arroliga AC, Ghamra ZW, Perez Trepichio A, Perez Trepichio P, Komara JJ, Smith A, et al. Incidence of ARDS in an adult population of northeast Ohio. Chest. 2002;121:1972–6.
- Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32.
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine: Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36:296–327.
- Azoulay É, Canet E, Raffoux E, Lengliné E, Lemiale V, Vincent F, et al. Dexamethasone in patients with acute lung injury from acute monocytic leukaemia. Eur Respir J. 2012;39:648–53.
- Martin M, Salim A, Murray J, Demetriades D, Belzberg H, Rhee P. The decreasing incidence and mortality of acute respiratory distress syndrome after injury. A 5-years observational study. J Trauma. 2005;59:1107–13.
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93.
- Azoulay E, Darmon M, Delclaux C, Fieux F, Bornstain C, Moreau D, et al. Deterioration of previous acute lung injury during neutropenia recovery. Crit Care Med. 2002;30:781–6.
- Rhee CK, Kang JY, Kim YH, Kim JW, Yoon HK, Kim SC, et al. Risk factors for acute respiratory distress syndrome during neutropenia recovery in patients with hematologic malignancies. Crit Care. 2009;13:R173.
- Azoulay E, Thiéry G, Chevret S, Moreau D, Darmon M, Bergeron A, et al. The prognosis of acute respiratory failure in critically ill cancer patients. Medicine. 2004;83:360–70.
- Depuydt PO, Benoit DD, Roosens CD, Offner FC, Noens LA, Decruyenaere JM. The impact of the initial ventilatory strategy on survival in hematological patients with acute hypoxemic respiratory failure. J Crit Care. 2010;25:30–6.
- Soares M, Salluh JI, Azoulay E. Noninvasive ventilation in patients with malignancies and hypoxemic acute respiratory failure: a still pending question. J Crit Care. 2010;25:37–8.