Abstract
Background
Wilms' tumor (WT1) gene overexpression has been reported in the majority of acute myeloid leukemia (AML) patients at diagnosis and has been evaluated as prognostic and minimal residual disease (MRD) marker.
Patients and methods
WT1 overexpression was evaluated in 68 adult AML patients at diagnosis and at the end of induction using quantitative real-time polymerase chain reaction (PCR).
Results
No significant associations were encountered between WT1 overexpression at diagnosis and other prognostic factors. Complete remission (CR) was achieved in 74% of the patients with WT1 overexpresssion compared to 80% of patients with normal levels (P = 0.5). No significant associations were encountered between WT1 overexpression at diagnosis and disease-free survival (DFS) or overall survival (OS) (P = 0.6 and 0.3, respectively). At the end of induction, the median duration of DFS in patients achieving ≥2 log reduction was not reached compared to only 5 months (range: 2.1–7.9 months) in those attaining <2 log reduction (P = 0.2). The median duration of OS in patients achieving ≥2 log reduction was 13 months (range: 0–33.3 months) compared to 7.5 months (5.4–9.6 months) in those attaining <2 log reduction (P = 0.2). The survival at 1 year in patients achieving ≥2 log was double the group with <2 log reduction (67% compared to 33%).
Conclusion
Our results, although not reaching the level of significance, probably due to the small sample size, still suggest that the early assessment of WT1 transcript level at the end of induction in patients in CR may have a prognostic significance on clinical outcome and may thus be a useful marker for risk stratification especially in patients lacking disease-specific marker.
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia in adults.Citation1 The genetic abnormalities associated with significant proportion of AML cases provide unique markers that can be used for risk stratification and minimal residual disease (MRD) monitoring.
Wilms' tumor 1 (WT1) gene is involved in normal and malignant hematopoiesis.Citation2 It encodes a transcription factor important for normal cellular development and cell survival.Citation3 Its overexpression has been reported in the majority of AML patients and has been evaluated as prognostic and MRD marker.Citation4–Citation6
Studies investigating the clinical value of WT1 transcript detection at diagnosis produced conflicting results. While few investigators draw a clear correlation between WT1 overexpression at initial diagnosis and poor prognosis,Citation7,Citation8 others could not confirm these findings.Citation6,Citation9,Citation10 However, WT1 antigen elicits a cytotoxic T lymphocyte activity and is gaining increasing attention as a therapeutic target molecule due to its common expression in acute leukemias and its involvement in cell proliferation.Citation11
Among AML patients who attain complete remission (CR), only about one-third of these patients remain free of disease for more than 5 years.Citation12 Detection and monitoring of MRD is an important prognostic factor in acute leukemia, quantification of MRD after induction represents a powerful predictor of disease-free survival (DFS) and overall survival (OS)Citation13,Citation14 and may provide the basis for risk stratification and patient-tailored therapy. In cases lacking a leukemia-specific MRD marker, quantification of gene overexpression could provide a precise measurement of disease response.Citation15 WT1 gene overexpression has been investigated in MRD detection in AML patients, data suggest that early reduction in WT1 transcript after induction chemotherapy may give an indication of the quality of response.Citation5,Citation16–Citation20
The aim of our study is to evaluate the WT1 gene expression as a potential prognostic marker in AML patients at diagnosis and its implication as a marker for MRD at the end of induction chemotherapy.
Patients and methods
Sixty-eight newly diagnosed adult AML patients who presented to the Medical Oncology Department of the National Cancer Institute, Cairo University were included in this study. Written consent was obtained from the patients and the protocol was approved by the Institution Research Board. The diagnosis of AML was established according to the morphological and cytochemical criteria of the French-American-British classification and immunophenotyping.
The expression level of WT1 was determined in bone marrow samples of patients at diagnosis and 10 normal controls using real-time quantitative polymerase chain reaction (PCR).
The frequency of WT1 overexpression and its correlation with known prognostic factors was determined. At the end of induction (day 28), the impact of WT1 transcript reduction in patients who attained CR on clinical outcome was analyzed. Bone marrow samples from healthy donors were used as control to define the normal range of WT1 expression in healthy subjects.
Patients received induction with 3 and 7 regimen combing daunorubicin 45 mg/m2 intravenous days 1–3 and cytosine arabinoside100 mg/m2 by continuous infusion from days 1–7 as an induction regimen. Evaluation of response was carried out at the end of induction. Postremission therapy was risk stratified with additional four cycles of high-dose cytosine arabinoside with mitoxantrone (HAM regimen). Last follow-up was the date of referral to transplantation for transplanted patients.
Bone marrow samples were collected in sterile EDTA tubes. Mononuclear cells were obtained using Ficoll-Hypaque density centrifugation. RNA was isolated using QIAamp RNA Blood Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The synthesis of cDNA from 1 µg of RNA was performed in 20 µl reaction using random hexamer according to the manufacturer's instructions using high capacity cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA).
Quantitative estimation of WT1 was performed by real-time quantitative PCR using the Light Cycler Instrumenmt (Roche, Germany). WT1 copy number was measured using TaqMan universal PCR Master Mix provided by Roche. Standards for ABL and WT1, primers and probes were provided by WT1 ProfileQuant Kit (ENL) (Ipsogen, Marseille, France). The fluorescent probe was labelled with reporter dye at 5′ end and with quencher dye at 3′ end. Standard curves were calculated for ABL using three dilutions of ABL plasmid (105, 104, and 103 copies in 5 µl) and for WT1 using five dilutions of WT1 plasmid (106, 105, 103, 102, and 101 copies in 5 µl).
TaqMan universal PCR Master Mix was used according to the manufacturer's instructions. Reactions (20 µl each) were prepared using 4 µl 5× LightCycler TaqMan Master Mix, 0.8 µl 25× Ipsogen primers and probes and 5 µl of cDNA (sample, standard, or control). The volume was adjusted to 20 µl using nuclease-free water. The reaction protocol proceeded as follows: 95°C for 10 minutes to activate the Taq DNA polymerase, followed by 95°C for 10 seconds, 60°C for 1 minute, steps 2 and 3 were repeated for 45 cycles, followed by 45°C for 1 minute. Fluorescence was measured using F1 channel. Results were calculated using LightCycler software. WT1 transcript values were normalized with respect to the number of ABL transcript and expressed as WT1 copy number every 104 copies of ABL.
Results
WT1 expression at diagnosis
The frequency of WT1 overexpression at diagnosis and its correlation with other prognostic factors and clinical outcome were evaluated in a cohort of 68 newly diagnosed AML patients. The median age was 31 years (range 18–76). Male-to-female ratio was 1:1.3. The median expression level of WT1 transcript in AML patients was 16.650 (range 0–630.730) copies/104 ABL copies compared to a median of 24 copies/104 ABL copies (0–130) copies/104 ABL copies in normal controls. A WT1 level at least twice the maximum assessed in healthy controls (260 copies/104 ABL copies) was defined as WT1 overexpression. Similarly, values above 250 copies/104 ABL copies were considered as WT1 overexpression by ELN (European LeukemiaNet) WT1 assay.Citation19 The WT1 transcript was overexpressed in 51/68 (75%) of patients. Patient characteristics according to WT1 expression are mentioned in . No significant associations were encountered between WT1 over expression and prognostic factors including age, total leukocyte count, Blast% (). FAB classification showed no significant difference in WT1 expression in combined FAB subtypes compared to M4 and M5 (P = 1.0). Karyotype analysis was available for 15 patients at diagnosis with the following frequency: t(8;21) in three, t(15;17) in five, inv16 in one, and six patients had normal karyotype; the number was low to draw a statistical analysis.
Table 1. Characteristics of AML patients according to WT1 expression
To investigate the relationship between WT1 expression and clinical outcome, we studied response to chemotherapy and found the CR in 45/59 (76%) of evaluable patients; 29/39(74%) of patients with WT1 overexpression compared to 16/20 (80%) of patients with normal levels (P = 0.5) ().
Table 2. Clinical outcome of AML patients according to WT1 expression
During follow-up period, no significant differences were encountered in WT1 transcript level at diagnosis between patients who persisted in CR and those who relapsed. However, the median duration of DFS was shorter in patients overexpressing the gene compared to the other group (7.5 versus 11.5 months), (P = 0.6). Regarding OS, the median survival in patients overexpressing the gene was less than half the other group (7.5 versus 17 months), yet the difference was not statistically significant, P = 0.3. The survival at 1 year was 45% in the group overexpressing the gene compared to 66% in the other group.
WT1 expression at the end of induction
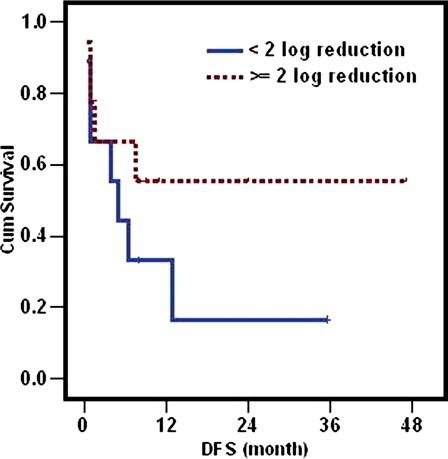
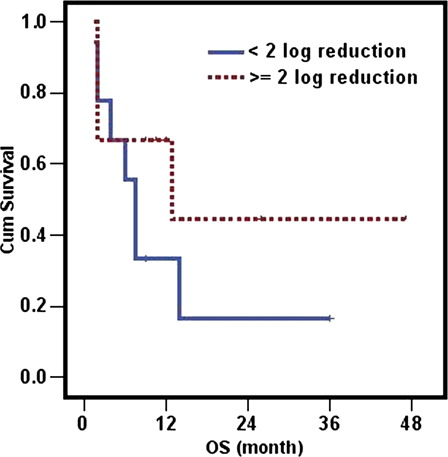
Assessment of MRD at the end of induction was performed using 2 log reduction according to the study conducted by the European Leukemia Net (ELN).Citation19 The impact of WT1 transcript reduction at the end of induction was evaluated in 18 patients in CR in whom the initial level of the transcript at diagnosis was sufficiently high to allow the follow-up using 2 log reduction. The median duration of DFS in patients attaining ≥2 log reduction was not reached whereas it was only 5 months (range: 2.1–7.9 m) in patients with <2 log reduction (P = 0.2) (). The median duration of OS in patients attaining ≥2 log reduction after induction was 13 months (range: 0–33.3) compared to 7.5 months (range: 5.4–9.6) in patients with <2 log reduction. Although the median duration was longer in the first group compared to the other (13 versus 7.5 months), yet the difference was not significant (P = 0.26) (). Patients achieving ≥2 log reduction had 1 year probability of survival double the other group (67 compared to 33%) ().
Discussion
AML is the most common acute leukemia in adults. WT1 overexpression in a significant proportion of AML cases provides unique marker that could be used for risk stratification and to monitor MRD in the majority of patients.
In the present study, the expression levels of WT1 were evaluated in a cohort of AML patients at diagnosis and in follow-up samples at the end of induction. WT1 levels were correlated to the clinical outcome of the disease. In our cohort, the WT1 transcript was overexpressed in 75% of AML patients at diagnosis. WT1 is reported to be overexpressed in approximately 70–90% of AML patients.Citation4,Citation5,Citation21 No significant associations were encountered between WT1 overexpression at diagnosis and other prognostic factors including age, total leukocyte count, and Blast percentage. This was in accordance with the previous studies who could not find an association between the gene overexpression and prognostic factors.Citation6,Citation9,Citation10,Citation17,Citation22 FAB classification showed no statistical difference in WT1 expression in combined FAB subgroups compared to M4 and M5 in accordance with some studies.Citation5,Citation6,Citation16,Citation23 On the contrary, Weisser et al.Citation17 and othersCitation4,Citation7,Citation12,Citation21,Citation24 found significant lower level in M5 subtype being more differentiated compared to more undifferentiated subtypes.
No observed significant difference in CR in patients overexpressing the gene compared to those without overexpression. This was similar to findings of Schmid et al.,Citation9 Barragan et al.Citation8 and Cilloni et al.,Citation6 who reported no difference in WT1 transcript at diagnosis in patients resistance compared to responders to chemotherapy.
Our study reported no association between WT1 overexpression and clinical outcome. The prognostic impact of WT1 level at diagnosis on clinical outcome is controversial, while some studies could not find a significant association between overexpression of the gene and DFS and OS,Citation5,Citation6,Citation9,Citation10,Citation12,Citation17,Citation19,Citation20,Citation24,Citation25 other data reported worse outcome with WT1 overexpression.Citation4,Citation7,Citation8,Citation16,Citation22,Citation26,Citation27
However, WT1 is gaining increasing attention as a therapeutic target molecule due to its common expression in acute leukemias and its involvement in cell proliferation.Citation11 It possesses immunogenetic properties and has been successfully tested as a target for antileukemic vaccine.Citation28,Citation29
Relapse remains one of the greater challenges in treating AML. Evaluation of MRD is important to assess quality of response after induction therapy.Citation15 Early identification of patients in CR at high risk of relapse after induction therapy allows modification of post-remission therapy including intensification of chemotherapy or stem cell transplantation. However, more than 50% of patients with AML lack a known chromosomal abnormality or genetic lesions suitable for MRD determination. The frequent overexpression of WI1 gene in AML makes it a good candidate for MRD quantification. Our MRD study was based on early assessment of reduction in the transcript level after induction treatment in patients attaining CR. Although in our cohort patients achieving ≥2 log reduction in the transcript at the end of induction had longer DFS, OS and a probability of survival at 1 year double the group achieving <2 log reduction, yet the difference was not statistically significant probably because of the small number of patients.
This strongly support the data of Ostergaard et al.,Citation5 who found that <2 log reduction in WT1 levels after induction therapy was associated with higher risk of relapse. Similarly, Cilloni et al.Citation19 reported reduced risk of relapse with ≥2 log reduction after the first cycle of chemotherapy. This also goes with Garg et al.,Citation16 who found that a level lower than 103 copies WT1/105 ABL copies after the induction was associated with favorable prognosis and correlated with significant better DFS and OS, also Ommen et al.Citation18 reported that WT1 level above normal after first remission was an independent prognostic factor regarding both DFS and OS. This was in line with Nowakowska-Kopera et al.Citation20 who reported an inverse correlation between high WT1 expression level after the induction chemotherapy and survival, and Gianfaldoni et al.,Citation30 who reported an association between early decrease of WT1 transcript level and better outcome. Also in pediatrics, Lapillonne et al.Citation24 found that WT1 evaluation after the first course of induction treatment represents the ideal tool to identify acute leukemia patients at high risk of relapse. On the other hand, Schmid et al.Citation9 and Gaiger et al.Citation10 failed to show correlation between WT1 level post-remission and clinical outcome while Weisser et al.Citation17 did not report prognostic significance of WT1 level 2 months post-induction.
Serial monitoring of WT1 transcript level is helpful, especially in patients with low WT1 expression at diagnosis not allowing follow-up using 2 log reduction. Rising levels of WT1 usually precedes clinical relapse in a significant proportion of patients.Citation5,Citation6,Citation16,Citation20
Although our results are not reaching the level of significance, which is probably due to the small sample size, it still suggests that early assessment of the WT1 transcript level at the end of induction in patients who attained CR may have prognostic significance on clinical outcome and may thus be a useful marker for risk stratification especially in patients lacking disease-specific marker. However, its applicability must be evaluated in a larger cohort of patients.
Acknowledgment
This work was funded by Cairo University.
References
- Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–62.
- Ariyaratana S, Loeb DM. The role of the Wilms tumour gene (WT1) in normal and malignant haematopoiesis. Expert Rev Mol Med. 2007;9(14):1–17.
- Sugiyama H. WT1 (Wilms' tumor gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol. 2010;40(5):377–87. Epub 2010 Apr 15.
- Inoue K, Sugiyama H, Ogawa , Nakagawa M, Yamagami T, Miwa H, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood 1994;84:3071–9.
- Østergaard M, Olesen LH, Hasle H, Kjeldsen E, Hokland P. WT1 gene expression: an excellent tool for monitoring minimal residual disease in 70% of acute myeloid leukaemia patients - results from a single-centre study. Br J Haematol. 2004;125(5):590–600.
- Cilloni D, Messa F, Arruga F, Defilippi I, Gottardi E, Fava M, et al. Early prediction of treatment outcome in acute myeloid leukaemia by measurement of WT1 transcript levels in peripheral blood samples collected after chemotherapy. Haematologica 2008;93:921–4.
- Bergmann L, Miething C, Maurer U, Brieger J, Karakas T, Weidmann E, et al. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood 1997;90(3):1217–25.
- Barragan E, Cervera J, Bolufer P, Ballester S, Martín G, Fernández P, et al. Prognostic implications of Wilms’ tumor gene (WT1) expression in patients with de novo acute myeloid leukemia. Haematologica 2004;89(8):926–33.
- Schmid D, Heinze G, Linnerth B, Tisljar K, Kusec R, Geissler K, et al. Prognostic significance of WT1 gene expression at diagnosis in adult de novo acute myeloid leukemia. Leukemia 1997;11(5):639–43.
- Gaiger A, Schmid D, Heinze G, et al. Detection of the WT1 transcript by RT-PCR in complete remission has no prognostic relevance in de novo acute myeloid leukemia. Leukemia 1998;12(12):1886–94.
- Busse A, Gökbuget N, Siehl JM, Hoelzer D, Schwartz S, Rietz A, et al. Wilms' tumor gene 1 (WT1) expression in subtypes of acute lymphoblastic leukemia (ALL) of adults and impact on clinical outcome. Ann Hematol. 2009;88(12):1199–205.
- Miyawaki S, Hatsumi N, Tamaki T, Naoe T, Ozawa K, Kitamura K, et al. Prognostic potential of detection of WT1 mRNA level in peripheral blood in adult acute myeloid leukemia. Leuk Lymphoma 2010;51(10):1855–61.
- Kern W, Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T. Prognostic impact of early response to induction therapy as assessed by multiparameter flow cytometry in acute myeloid leukemia. Haematologica 2004;89(5):528–40.
- Buccisano F, Maurillo L, Del Principe MI, Del Poeta G, Sconocchia G, Lo-Coco F, et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood 2012;119(2):332–41.
- Buccisano F, Maurillo L, Spagnoli A, Del Principe MI, Ceresoli E, Lo Coco F, et al. Monitoring of minimal residual disease in acute myeloid leukemia. Curr Opin Oncol. 2009;21(6):582–8.
- Garg M, Moore H, Tobal K, Liu Yin JA. Prognostic significance of quantitative analysis of WT1 gene transcripts by competitive reverse transcription polymerase chain reaction in acute leukaemia. Br J Haematol. 2003;123(1):49–59.
- Weisser M, Kern W, Rauhut S, Schoch C, Hiddemann W, Haferlach T, et al. Prognostic impact of RT-PCR-based quantification of WT1 gene expression during MRD monitoring of acute myeloid leukemia. Leukemia 2005;19(8):1416–23.
- Ommen HB, Nyvold CG, Braendstrup K, Andersen BL, Ommen IB, Hasle H, et al. Relapse prediction in acute myeloid leukaemia patients in complete remission using WT1 as a molecular marker: development of a mathematical model to predict time from molecular to clinical relapse and define optimal sampling intervals. Br J Haematol. 2008;141(6):782–91.
- Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27(31):5195–201.
- Nowakowska-Kopera A, Sacha T, Florek I, Zawada M, Czekalska S, Skotnicki AB. Wilms' tumor gene 1 expression analysis by real-time quantitative polymerase chain reaction for monitoring of minimal residual disease in acute leukemia. Leuk Lymphoma 2009;50(8):1326–32.
- Cilloni D, Gottardi E, De Micheli D, Serra A, Volpe G, Messa F, et al. Quantitative assessment of WT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients. Leukemia 2002;16(10):2115–21.
- Trka J, Kalinova M, Hrusak O, Zuna J, Krejci O, Madzo J, et al. Real time quantitative PCR detection of WT1 gene expression in children with AML: prognostic significance, correlation with disease status and residual disease detection by flow cytometry. Leukemia 2002;16:1381–9.
- Noronha SA, Farrar JE, Alonzo TA, Gerbing RB, Lacayo NJ, Dahl GV, et al. WT1 expression at diagnosis does not predict survival in pediatric AML: a report from the Children's Oncology Group. Pediatr Blood Cancer 2009;53(6):1136–9.
- Lapillonne H, Renneville A, Auvrignon A, Flamant C, Blaise A, Perot C, et al. High WT1 expression after induction therapy predicts high risk of relapse and death in pediatric acute myeloid leukemia. J Clin Oncol. 2006;24(10):1507–15.
- Yanada M, Terakura S, Yokozawa T, Yamamoto K, Kiyoi H, Emi N, et al. Multiplex real-time RT-PCR for prospective evaluation of WT1 and fusion gene transcripts in newly diagnosed de novo acute myeloid leukemia. Leuk Lymphoma 2004;45(9):1803–8.
- Inoue K, Ogawa H, Yamagami T, Soma T, Tani Y, Tatekawa T, et al. Long-term follow-up of minimal residual disease in leukemia patients by monitoring WT1 (Wilms tumor gene) expression levels. Blood 1996;88:2267–78.
- Gray JX, McMillen L, Mollee P, Paul S, Lane S, Bird R, et al. WT1 expression as a marker of minimal residual disease predicts outcome in acute myeloid leukemia when measured post-consolidation. Leuk Res. 2012;36(4):453–8.
- Ochsenreither S, Fusi A, Busse A, Bauer S, Scheibenbogen C, Stather D, et al. Wilms Tumor Protein 1″ (WT1) peptide vaccination-induced complete remission in a patient with acute myeloid leukemia is accompanied by the emergence of a predominant T-cell clone both in blood and bone marrow. J Immunother. 2011;34(1):85–91.
- Van Driessche A, Berneman ZN, Van Tendeloo VF. Active specific immunotherapy targeting the Wilms' Tumor Protein 1 (WT1) for patients with hematological malignancies and solid tumors: lessons from early clinical trials. Oncologist 2012;17(2):250–9.
- Giacomo G, Francesco M, Vanessa P, Giovanni L, Sara B, Alberto B, et al. Early reduction of WT1 transcripts during induction chemotherapy predicts for longer disease free and overall survival in acute myeloid leukemia. Haematologica 2010;95(5):833–6.