Abstract
Objective
To evaluate the efficacy of imatinib administration before and/or after allogeneic hematopoietic stem cell transplantation (allo-HSCT) for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
Method
Patients with imatinib therapy time exceeding 30 days pre-/post-transplant were screened in our data. Imatinib was used in induced or consolidated chemotherapy pre-transplant, or maintenance therapy after 60 days post-transplant (therapy time was less than 180 days) regardless of the molecular status of the disease.
Results
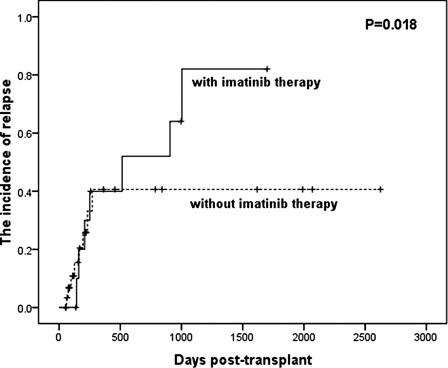
Sixty-nine patients with Ph+ ALL were enrolled in the retrospective analysis. Forty-four patients received imatinib therapy, including 24 pre-transplant, 9 post-transplant, and 11 both pre- and post-transplant. With a median follow-up time of 395 days (range, 55–2762 days) post-transplant, 3-year estimated overall survival was 62.3 ± 16.6, 40.0 ± 21.9, 41.7 ± 22.2, and 25.9 ± 11.4%, respectively (P = 0.221), and disease-free survival (DFS) was 53.6 ± 17.9, 20.0 ± 17.9, 33.3 ± 25.5% and 23.6 ± 11.4%, respectively (P = 0.421), in patients with imatinib therapy pre-transplant, post-transplant, both pre- and post-transplant, neither pre- nor post-transplant. The incidence of relapse at 3 year for patients with imatinib therapy post-transplant (n = 20) was 63.6%, comparing with 24.2% (P = 0.018) in patients without imatinib therapy post-transplant (n = 49). The ratio of CD4+CD25+Foxp3+ cells in blood was significantly higher at 30 and 60 days after imatinib therapy than that at the time of pre-imatinib in 20 patients (P = 0.019 and 0.001, respectively).
Conclusions
Application of imatinib pre-transplant might have benefited for patients with Ph+ ALL. Whether administration of imatinib, regardless of the molecular status of the disease post-transplant increases relapse, is a worthy goal for further study.
Introduction
Philadelphia chromosome (Ph) is a reciprocal chromosomal translocation t(9;22)(q34;q11), which leads to the formation of the BCR/ABL oncogene. The product of this fusion gene is a constitutively active protein tyrosine kinase, which plays a critical role in leukemogenesis. Ph is the most frequent cytogenetic abnormality in adult acute lymphoblastic leukemia (ALL), with an overall incidence of 20–40%, characterized by poor outcome.Citation1–Citation3 Although induction remission rate of Ph+ ALL can reach 50–80%, most patients face high relapse and low survival after recurrence.Citation4,Citation5
Imatinib is a potent selective inhibitor of the BCR/ABL protein tyrosine kinase. Since 1999, imatinib was approved for human use as a competitive inhibitor directed toward BCR/ABL fusion gene found in both CML and Ph+ ALL. So far, imatinib has replaced allo-HSCT for the first-line treatment of Ph+ chronic myeloid leukemia. Meanwhile, imatinib has also become an integral part of front-line therapy for Ph+ ALL, with remission rates exceeding 90% irrespective of whether imatinib is given alone or combined with chemotherapy.Citation6 Treatment outcome with imatinib-based regimens has improved the remission rate, but most patients who do not undergo allogeneic hematopoietic stem cell transplantation (allo-HSCT) eventually relapse.Citation1,Citation2,Citation7 Allo-HSCT is one promising mean that can cure Ph+ ALL, especial in the early course of the disease, but the long-term survival only ranged from 21 to 57%.Citation8–Citation11 Some reports showed that pre-transplant imatinib therapy could improve disease-free survival (DFS) for Ph+ ALL; however, whether post-transplant imatinib administration might improve DFS remains contentious.Citation12–Citation14 In this report, we retrospectively evaluated the efficacy of imatinib administration pre-and/or post-HSCT for patients with Ph+ ALL.
Methods
Patient
Forty-four patients with Ph+ ALL, who underwent allo-HSCT from 2001 to 2011 at Nanfang hospital, Southern Medical University, were enrolled in this analysis. The diagnosis of Ph+ ALL was based on the presence of the t(9;22) on standard karyotype analysis and/or fluorescence in situ hybridization. The majority of patients underwent induction chemotherapy with the VDLP (vincristine, daunorubicin, l-asparaginase, and prednisone) regimen, whereas other patients received variations of this regimen that also included high-dose cytarabine, 6-mercaptopurine, idarubicin, and/or cyclophosphamide or the hyper-CVAD (A: fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone. B: high-dose cytarabine and methotrexate) regimen. This retrospective report was performed in accordance with the modified Helsinki Declaration and approved by the ethical review boards before data analysis. All donors, recipients and/or guardians provided written informed consent.
Imatinib therapy
The indrawing criteria of imatinib therapy were defined by more than a month of imatinib administration before or after transplantation. Patients who relapsed in hematology or cytogenetics post-transplant followed by the imatinib therapy were not enrolled in this analysis. The decision whether to initiate imatinib therapy pre-transplant was made by the attending physician other than typically the standard of practice at the time. Imatinib therapy post-transplant was decided by the transplant physician, and imatinib therapy was initiated within 60 to 90 days after transplantation for the eligible patients if their peripheral absolute neutrophil counts were above 1.0 × 109/l and the platelet counts were more than 50.0 × 109/l, regardless of the levels of BCR/ABL transcript. Either before or after transplantation, imatinib dose was 400–600 mg/day, which was stopped when neutrophil was below 0.5 × 109/l or the platelet was below 20.0 × 109/l, and imatinib was reapplied when neutrophil was above 0.5 × 109/l and the platelet was above 30.0 × 109/l. Generally, imatinib therapy time was less than 180 days post-transplant.
Transplantation
Of 69 recipients, 35 received related donor transplants and 34 unrelated donor transplants. Of 35 patients undergoing related donor transplants, 28 cases were human leukocyte antigen (HLA) matched, 5 were 2/10 HLA-alleles mismatched, and 2 were 3/10 HLA-alleles mismatched. Of the 34 unrelated donor transplants, 15 cases were HLA matched, 13 were 1/10 HLA-allele mismatched, 3 were 2/10 HLA-alleles mismatched, and 3 were 3/10 HLA-alleles mismatched. Total body irradiation (TBI)+cyclophosphamide (CY) conditioning regimen (TBI 4.5 Gy/day, −5, −4 days; CY 60 mg/kg/day, −3, −2 days) was used for patients with complete remission (CR) before transplantation. TBI+CY+etoposide (VP-16) conditioning regimen (VP-16, 10–15 mg/kg/d, −3, −2 days), or fludarabine (30 mg/m2/day, −10 to −6 days)+cytarabine (2.0 g/m2/day, −10 to −6 days) plus TBI+CY+VP-16 conditioning were administered in patients with none-CR (nCR) before transplantation.Citation15
Graft-versus-host disease prophylaxis and treatment
Cyclosporine A (CsA) alone or CsA plus methotrexate (MTX) (on days +1 and +3) were administered in patients with nCR undergoing HLA-matched sibling donor transplantation, and CsA plus MTX (on days +1, +3 and +6) were administered in patients with CR undergoing HLA-matched sibling donor transplants for graft-versus-host disease (GVHD) prophylaxis. CsA+MTX+antithymocyte globulin (ATG, total ATG doses of 6–10 mg/kg, on days −3 to −1 or −4 to 0) and/or mycophenolate mofetil (MMF, 0.5 g/day, twice daily, on days 0 to +28) were used in patients undergoing HLA-mismatched related and unrelated donor transplants. Methylprednisolone (1–2 mg/kg/day) was used to treat acute GVHD (aGVHD). ATG or ATG combined with CD25 monoclonal antibody and other immunodepressants were used to treat glucocorticosteroid-resistant aGVHD. Corticosteroids and CsA were used initially to treat chronic GVHD (cGVHD) and were used in combination with various immunosuppressive agents to treat cGVHD that was unresponsive to initial therapy.Citation15
CsA withdrawal and donor lymphocyte infusion (DLI)
In order to induce graft versus leukemia (GVL), all potential patients accepted early rapid tapering of prophylactic immunosupressants therapy for GVHD and DLI during the early stage after transplantation. Before June 2008, CsA was withdrawn rapidly in a stepwise fashion (i.e. total dose reduced by 20% per week) in the patients who had no aGVHD by day +30 post-transplant.Citation16 After June 2008, CsA was withdrawn in a stepwise manner (i.e. total dose reduced by 10% per week) in the patients who did not occur aGVHD by day +30 post-transplant, and G-CSF mobilized DLI (1.0 × 108/kg, once a month, four doses totally) would be used in the patients without II or above acute GVHD by day +60 days post-transplant. Once the patients developed GVHD after DLI, DLI would stop and methylprednisolone was added to the regimen.
Statistical analysis
The SPSS software package (SPSS, Chicago, IL, USA) was used to process all the data. Differences between characteristics were assessed by Kruskal–Wallis test. Overall survival (OS, DFS, and incidence of relapse were described according to Kaplan–Meier method. In the determination of DFS, death and relapse were considered as events. Differences between survival curves were assessed by the log rank test. Statistical significance was defined at the P less than or equal to 0.05 level.
Results
Patient's clinical and treatment characteristics
The median age at the time of transplants was 29.5 years (range 12–61). Twenty-four patients were female, and 45 were male. Twenty-seven patients had other chromosomal abnormalities other than Ph chromosome. The blast cells of 22 patients also expressed myeloid lineage. Thirty-six patients were in first CR (CR1), 9 in CR2 and 24 in nCR at the time of transplantation. Patient's clinical and treatment characteristics are showed in .
Table 1. Patient and treatment characteristics
Application of imatinib
Of 69 patients, 44 patients received imatinib therapy, including 24 patients received imatinib pre-transplant, 9 post-transplant, 11 both pre- and post-transplant, and 25 did not accept imatinib therapy. Totally, of 35 patients who received imatinib pre-transplant, 27 patients accepted imatinib therapy during the time of initiate induction chemotherapy, and 3 during the time of consolidation therapy, 5 patients accepted imatinib therapy during the time of refractory and relapse. Twenty-five of 27 patients obtained CR in patients who accepted imatinib therapy during the initiate induction and 4 CR in the 5 patients who accepted imatinib therapy during the time of refractory and relapse. The time of imatinib therapy pre-transplant was at a median of 98 days (range 33–256 days). Of 20 patients who received imatinib post-transplant, the imatinib therapy was initiated at a median of 67 days (range 60–89 days) post-transplant and the time of imatinib therapy was at a median of 78 days (range 34–178 days).
Engraftment
All patients achieved sustained donor engraftment. The median time of neutrophil engraftment was 13 days (range 10–21 days) and 13.5 days (range 10–20 days), respectively, in patients with imatinib therapy and without imatinib therapy pre-transplant (P = 0.893). The median time of platelet engraftment was 16 days (range 11–28 days) and 17 days (range 12–35 days) in patients with and without imatinib therapy pre-transplant (0.070).
GVHD and DLI administration
The incidence of grade 0-I and II-IV aGVHD including aGVHD after DLI were 44.9 and 55.1%, respectively. The incidence of cGVHD was 47.8%, and extensive cGVHD occurred in 13 patients (18.8%) (). Thirty-four patients who had not grade II or above aGVHD within 60 days post-transplant accepted DLI. After DLI, 18 of 34 patients developed grade II-IV aGVHD, 10 of 34 patients developed limit cGVHD. One patient who had DLI died of aGVHD.
Table 2. GVHD in the entire cohort
In order to exclude the effect of DLI on GVHD, the entire cohort was screened for 35 patients without DLI. The difference of incidence of aGVHD and cGVHD were not significant between patients with and without imatinib therapy pre-transplant (P = 0.554 and 0.970, respectively). Likewise, the incidence of aGVHD and cGVHD were also not significantly different between patients with or without imatinib therapy post-transplant (P = 0.586 and 0.826, respectively) ().
Table 3. GVHD in 35 patients without prophylactic DLI
Lymphocyte subset analysis in peripheral blood
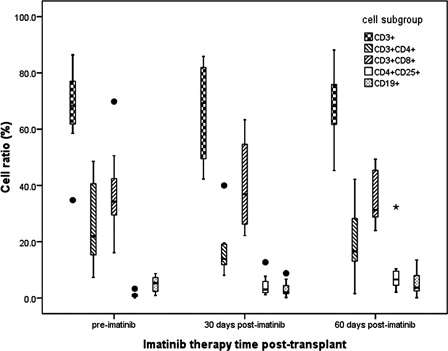
In post-transplant, flow cytometry was performed to detect lymphocyte subsets in peripheral blood in patients at the time point of pre-imatinib (before using imatinib), 30 days and 60 days (different time points were defined as within-subjects) after imatinib therapy (n = 20) and patients without imatinib therapy at the same period (n = 25) (two groups were defined as between-subjects). Repeated measures of general linear model showed that neither within-subjects nor between-subjects had significant differences in the ratio of blood CD3+ T cells (P = 0.096 and 0.368, respectively), CD3+CD4+T cells (P=0.058 and 0.079, respectively), CD3+CD8+ T cells (P = 0.235 and 0.310, respectively), and CD19+ B cells (P=0.108 and 0.328, respectively). The ratio of CD4+CD25+Foxp3+ T cells (Tregs) were significantly higher at 30 and 60 days after imatinib therapy than that in pre-imatinib (, P = 0.019 and 0.001, respectively). Compared with patients who did not receive imatinib therapy at the same period, the ratio of Tregs cells at 30 days and 60 days after imatinib therapy post-transplant were significantly increased (P=0.001). Linear analysis showed that the frequency of T-reg population and the survival time did not have a significant relationship (RCitation2 = 0.417, P = 0.124).
Risk factor
Univariate and multivariate analysis about the risk factors for survival and relapse are showed in . Univariate and multivariate analysis both showed that the CR status at transplant was significantly associated with better OS, DFS, and relapse. In contrast, age, the blasts with myeloid lineage expressing, imatinib therapy, HLA typing, donor type, conditioning regimen, and the occurrence of GVHD did not show any significant influence on the risk of survival and relapse.
Table 4. Univariate and multivariate analyses of risk factors
Survival
With a median follow-up time of 395 days (range, 55–2762 days) post-transplant, 32 patients were alive and 37 were dead. Causes of death included relapse (40.5%, n = 15), aGVHD (n = 8), cGVHD (n = 7), infections (n = 6), and thrombotic microangiopathy (n = 1). The estimated 3-year OS and DFS rates were 37.9±8.7, and 30.5±8.9%, respectively. The estimated 3-year OS was 62.3±16.6, 40.0±21.9, 41.7±22.2, and 25.9±11.4%, respectively (P = 0.221), and 3-year DFS was 53.6±17.9, 20.0±17.9, 33.3±25.5, and 23.6±11.4%, respectively (P = 0.421) in the patients with imatinib therapy pre-transplant, post-transplant, both pre- and post-transplant, neither pre- nor post-transplant. Regardless of imatinib pre-transplant, 3-year OS and DFS were 20.2±16.3 and 15.5±10.1% for patients received imatinib post-transplant (n = 20), respectively, compared with 38.5±10.8 and 36.0±10.5% for patients without imatinib therapy post-transplant (n = 49) (P = 0.691 and 0.884, respectively).
By the date when the data were analyzed, 19 patients have relapsed (27.5%) after transplantation. The incidence of relapse at 3 years was 14.3, 80.0, 50.0, and 31.6% in the patients with imatinib therapy pre-transplant, post-transplant, both pre- and post-transplant, neither pre- nor post-transplant, respectively. Regardless of imatinib therapy pre-transplant, the incidence of relapse at 3 years for patients with imatinib therapy post-transplant (n = 20) was 63.6%, comparing with 24.2% (P = 0.018) in patients without imatinib therapy (n = 49) post-transplant ().
Discussion
As an integral part of front-line induction therapy for Ph+ ALL, imatinib has been widely accepted now. Yanada et al.Citation17 reported that 80 adult patients with Ph+ ALL received imatinib during induction/consolidation therapy. CRs were observed in 77 of 80 (96.2%) patients and 1-year OS was 73.3% in patients receiving allo-HSCT as compared with 84.8% CR ratio in patients treated with chemotherapy alone. The similar results were also observed in our patients. In this entire cohort, 25 patients obtained CR in 27 patients (92.6%) who received imatinib during the time of initiate induction chemotherapy, and 4 patients CR in 5 patients who accepted imatinib during the time of refractory and relapse.
There is a general agreement that allo-HSCT is the only curative treatment available for adults with Ph+ ALL, but still with high relapse, resulting in less than 20% of DFS.Citation3,Citation10,Citation18 A growing body of clinical evidence indicates that application of imatinib pre-transplant can reduce the recurrence rate and improve long-term survival of Ph+ ALL patients after allo-HSCT.Citation13,Citation14 As a consequence, most ALL study groups consider imatinib-based treatment followed by allo-HSCT in CR1, to be the gold standard of first-line therapy for Ph+ ALL. The risk factor analysis of our cohort also confirmed that patients in CR followed by allo-HSCT had better outcome. In a prospective study, Mizuta et al.Citation19 reported that the 3-year OS and DFS post-transplants were 6% and 58%, respectively, for the imatinib cohort, and 44 and 37%, respectively, for the none-imatinib cohort. The 3-year incidences of relapse were 15.0 and 50.4%, respectively, in the imatinib and the none-imatinib cohort. In our cohort, 3-year OS and DFS were 62.3 ± 16.6 and 53.6 ± 17.9%, respectively, in patients with imatinib therapy pre-transplant, and 25.9 ± 11.4 and 23.6 ± 11.4%, respectively, in patients without imatinib therapy pre-transplant, which also confirmed that imatinib-based therapy followed by allo-HSCT was superior to that without imatinib therapy pre-transplant. Compared with related reports,Citation9,Citation10,Citation19,Citation20 our entire OS and DFS were relatively low. A reasonable interpretation of our low OS and DFS might be that there were additional chromosome abnormalities in majority, blasts with myeloid lineage expressing, and nCR status at the time of transplants in our cohort.
Currently, there is no evidence that imatinib has an adverse effect on transplant-related morbidity or mortality.Citation14,Citation17,Citation18 National Comprehensive Cancer Network had recommended that maintenance therapy following HSCT with the addition of a tyrosine kinase inhibitor (TKI) for Ph+ ALL. However, the effect of post-transplant imatinib administration on survival remains uncertain. Majority reports suggested that post-transplant imatinib administration might result in favorable survival, especially for ones with imatinib therapy according to minimal residual disease test (MRD).Citation14,Citation20–Citation23 In this reports, imatinib was used for patients regardless of the molecular status of the disease. To our surprise, our results showed that post-transplant imatinib therapy had increased the relapse and decreased OS and DFS. The interpretation of our results might be that, compared to other literature with MRD test,Citation14,Citation22 the duration of imatinib therapy in our center was relative longer, which might have made the appropriate imatinib therapy longer and led to immunosuppression,Citation24 which might have decreased GVL and increased the relapse. Imatinib, as a TKI, which inhibits the breakpoint cluster region-abelson tyrosine kinase of Ph+ hematological malignancies,Citation25 also have the same effect to tyrosine kinase in normal cells. Imatinib alter immunological characteristics of nature killer cells, T cells, antigen-presenting cell via affecting tyrosine kinase activity of normal cells.Citation4,Citation23,Citation26 This alteration performed as having inhibitory effects on immune functions post-transplant,Citation24 which was used to treat GVHD.Citation25 In order to illustrate the mechanism in our surprising result, the dynamic of lymphocyte subsets in peripheral blood was analyzed during the imatinib therapy post-transplant. We found that the ratio of Tregs cells was significantly increased at 30 and 60 days after imatinib therapy, compared with that in the same patients before imatinib therapy. Tregs cells suppress immune system to autoantigen and alloantigen response through an ‘active’ pattern and play an important role in maintaining the body's immune tolerance and balance, which effect on both GVHD and GVL.Citation15 However, we did not observe that aGVHD and cGVHD were significantly different in patients with or without imatinib therapy post-transplant. Due to our small sample, it fails to come to the conclusion that post-transplant administration of imatinib might result in increased relapse, which remains a question to the large number of prospective randomized study in future.
In summary, pre-transplant imatinib therapy has benefited for patients with Ph+ ALL undergoing allo-HSCT. Regardless of copies of BCR/ABL fusion gene, whether post-transplant imatinib administration is beneficial to patients with Ph+ ALL needs further study.
Acknowledgements
F.-H.Z. and Y.-W.L. contributed equally to this work. Q.-F.L. performed the research. F.-H.Z. and Y.-W.L. wrote the paper. X.Z., Y.Z., F.H. and Z.-P.F. analyzed the data. H.-S.Z., Q.-L.J. and J.S. designed the research study and contributed the informed consent.
References
- Preti HA, O'Brien S, Giralt S, Beran M, Pierce S, Kantarjian HM. Philadelphia-chromosome-positive adult acute lymphocytic leukemia: characteristics, treatment results, and prognosis in 41 patients. Am J Med. 1994;97:60–5.
- Gleissner B, Gökbuget N, Bartram CR, Janssen B, Rieder H, Janssen JW, et al. Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99:1536–43
- Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–97.
- Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–50.
- Thomas DA, Kantarjian H, Smith TL, Koller C, Cortes J, O'Brien S, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–30.
- Ottmann OG, Pfeifer H. Management of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Hematol Am Soc Hematol Educ Program. 2009;1:371–81.
- Wetzler M, Dodge RK, Mrozek K, Carroll AJ, Tantravahi R, Block AW, et al. Prospective karyotype analysis in adult acute lymphoblastic leukemia: the cancer and leukemia Group B experience. Blood. 1999;93:3983–93.
- Barrett AJ, Horowitz MM, Ash RC, Atkinson K, Gale RP, Goldman JM, et al. Bone marrow transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1992;79:3067–70.
- Chao NJ, Blume KG, Forman SJ, Snyder DS. Long-term follow-up of allogeneic bone marrow recipients for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1995;85:3353–4.
- Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113:4489–96.
- Lee S, Kim DW, Cho B, Kim YJ, Kim YL, Hwang JY, et al. Risk factors for adults with Philadelphia-chromosome-positive acute lymphoblastic leukaemia in remission treated with allogeneic bone marrow transplantation: the potential of real-time quantitative reverse-transcription polymerase chain reaction. Br J Haematol. 2003;120:145–53.
- Anderlini P, Sheth S, Hicks K, Ippoliti C, Giralt S, Champlin RE. Re: Imatinib mesylate administration in the first 100 days after stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:883–4.
- Carpenter PA, Snyder DS, Flowers ME, Sanders JE, Gooley TA, Martin PJ, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109:2791–3.
- Wassmann B, Pfeifer H, Stadler M, Bornhauser M, Bug G, Scheuring UJ, et al. Early molecular response to posttransplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood. 2005;106:458–63.
- Watanabe N, Narita M, Furukawa T, Nakamura T, Yamahira A, Masuko M, et al. Kinetics of pDCs, mDCs, gammadeltaT cells and regulatory T cells in association with graft versus host disease after hematopoietic stem cell transplantation. Int J Lab Hematol. 2011;33:378–90.
- Liu QF, Fan ZP, Zhang Y, Jiang ZJ, Wang CY, Xu D, et al. Sequential intensified conditioning and tapering of prophylactic immunosuppressants for graft-versus-host disease in allogeneic hematopoietic stem cell transplantation for refractory leukemia. Biol Blood Marrow Transplant. 2009;15:1376–85.
- Yanada M, Ohno R, Naoe T. Recent advances in the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Int J Hematol. 2009;89:3–13.
- Lee S, Kim YJ, Min CK, Kim HJ, Eom KS, Kim DW, et al. The effect of first-line imatinib interim therapy on the outcome of allogeneic stem cell transplantation in adults with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2005;105:3449–57.
- Mizuta S, Matsuo K, Yagasaki F, Yujiri T, Hatta Y, Kimura Y, et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia. 2011;25:41–7.
- Ottmann OG, Druker BJ, Sawyers CL, Goldman JM, Reiffers J, Silver RT, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. 2002;100:1965–71.
- Ram R, Storb R, Sandmaier BM, Maloney DG, Woolfrey A, Flowers ME, et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica. 2011;96:1113–20.
- Chen H, Liu KY, Xu LP, Liu DH, Chen YH, Shi HX, et al. Administration of imatinib in the first 90 days after allogeneic hematopoietic cell transplantation in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Chin Med J (Engl). 2011;124:246–52.
- Chen H, Liu KY, Xu LP, Liu DH, Chen YH, Zhao XY, et al. Administration of imatinib after allogeneic hematopoietic stem cell transplantation may improve disease-free survival for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. J Hematol Oncol. 2012;5:29.
- Iacobucci I, Lonetti A, Messa F, Cilloni D, Arruga F, Ottaviani E, et al. Expression of spliced oncogenic Ikaros isoforms in Philadelphia-positive acute lymphoblastic leukemia patients treated with tyrosine kinase inhibitors: implications for a new mechanism of resistance. Blood. 2008;112:3847–55.
- Gruber F, Mustjoki S, Porkka K. Impact of tyrosine kinase inhibitors on patient outcomes in Philadelphia chromosome-positive acute lymphoblastic leukaemia. Br J Haematol. 2009;145:581–97.
- Seggewiss R, Price DA, Purbhoo MA. Immunomodulatory effects of imatinib and second-generation tyrosine kinase inhibitors on T cells and dendritic cells: an update. Cytotherapy. 2008;10:633–41.