Abstract
Adipocytokines was stated to exert biological effect on tumor cells. Two adipokines, leptin and adiponectin in particular, have come to be recognized for their influence on tumor biology including leukemia. The prognostic effect of leptin and adiponectin concentrations in acute leukemia patients remains to be identified. This study was conducted on 80 acute leukemia patients: 35 acute myeloid leukemia (AML), 45 acute lymphoid leukemia (ALL), and 20 controls of matched age and sex. Leptin and adiponectin were assayed by enzyme-linked immunosorbent assay at diagnosis. Serum leptin levels were significantly higher in ALL patients, and significantly lower in AML patients when compared with normal controls (P = 0.01, P = 0.04 respectively). On the other hand, serum adiponectin levels were significantly lower in AML and ALL patients as compared with normal controls (P = 0.00 for both). No significant differences exist regarding body mass index between acute leukemia patients and normal controls (P > 0.05). Correlation studies revealed that there were significant negative correlations between serum adiponectin levels and bone marrow (BM) blast cells and serum lactic dehydrogenase (sLDH) in acute leukemia groups (r 0.542, P < 0.01, r 0.699, P < 0.001, respectively). Regarding serum leptin levels there were positive significant correlations with BM blast cells (r 0.74, P < 0.01), total WBC counts (r = 0.59, P < 0.05), sLDH (r 0.738, P < 0.01) in ALL group; and significant negative correlations with BM blast cells (r 0.542, P < 0.01) and sLDH in the AML group. Adipocytokines may represent a new non-invasive biomarker in acute leukemia patients. Estimation of adiponectin and leptin serum levels at acute leukemia diagnosis could also be considered as a prognostic marker, which will be used in acute leukemia stratification.
Keywords:
Introduction
Acute leukemia is characterized by clonal proliferation of the hematopoietic progenitor cells and accumulation of immature cells. The overall long-term free survival after intensive chemotherapy is less than 50%, but the prognosis can be improved by allogeneic stem cell transplantation for subsets of younger patients. The further investigation of these new treatment strategies will require detailed knowledge about the regulation of leukemic hematopoiesis by the cytokine network in the bone marrow (BM).Citation1 Both leukemia and its treatment can have a severe catabolic effect. Even, when well and in remission patients with cancer are severely catabolic even in remission state.Citation2
Adipokines are a group of novel and highly active molecules that are abundantly secreted by adipocytes, and act at both the local and systemic levels. They have attracted considerable interest due to their potential role in the development of cancer as a risk factor. Adipokines have been shown to regulate the survival, proliferation, differentiation and function of normal hematopoietic and leukemic cells.Citation3 Two adipokines, leptin and adiponectin in particular, have come to be recognized for their influence on tumor biology.Citation4
Leptin is a 16-kDa peptide hormone predominantly produced by white adipose tissue. The main function of leptin in the human body is the regulation of energy expenditure and control of appetite. Serum level of leptin reflects the amount of energy stored in the adipose tissue and is in proportion to body fat mass.Citation5
Leptin appears to play an important role in immunity and hematopoiesis. Leptin exerts proliferative, anti-apoptotic, and differentiating effects on hematopoietic neoplastic cells. Marrow adipocytes are a significant source of leptin in the BM.Citation6 Leptin induces its action through receptors, which was reported to be expressed in leukemic cells from patients with acute myeloblastic leukemia (AML), higher expression in blast crisis than chronic phase in CML were reported. They are expressed weakly in acute lymphoid leukemia (ALL) blasts, but not in CLL cells.Citation7
Adiponectin, an adipocyte-derived secretory protein, is a 30-kDa complement C1q-related protein. Adiponectin circulates as several multimeric species, including a high molecular weight form thought to be the most clinically relevant. Serum levels of adiponectin are markedly decreased in individuals with visceral obesity and states of insulin resistance, such as type 2 diabetes mellitus and atherosclerosis.Citation8 In addition to the relations between adipocytokines and obesity or diabetes, numerous other functions of these hormones in the human body have been identified, including potential roles in the regulation of inflammation, angiogenesis and tumor growth. Disturbances in the production of adipocyte-derived hormones may thus represent a new link explaining the well-known association between obesity and increased the prevalence of malignancies.Citation9
The aim of the current study is to assess the levels of leptin and adiponectin in acute leukemia, and to assess their prognostic roles.
Patients and methods
The present study was conducted on 80 patients with untreated acute leukemia (60 males and 40 females), age ranged from 33 to 75 years, and 20 controls of matched age and sex. They were recruited from Mansoura Oncology Center. The study was approved by the research ethics committee and consents were obtained from patients in the study.
Group I: Includes 35 patients with ALL before start of therapy
Group II: Includes 45 AML patients before start of therapy
Group III: Includes 20 healthy controls of matched age and sex.
| •. | Patients with febrile neutropenia, sepsis, any organ failure, with hypertension or diabetes were excluded. | ||||
| •. | Body mass index (BMI) was calculated by dividing body weight (kg) by square height (m2). We dichotomized BMI as BMI < 25 (non-overweight and non-obese) and BMI > 25 (overweight and obese). | ||||
Specimen collection:
| •. | Blood samples were collected for complete blood count (1 cm EDTA) from three groups. | ||||
| •. | Blood were collected from group I and group II (2 cm EDTA) for immunophenotyping using flow cytometry. | ||||
| •. | Two milliliters serum samples were collected for serum leptin, adiponectin, and serum lactic dehydrogenase (sLDH) estimation from the three studied groups. Blood samples were obtained after an overnight fast. | ||||
| •. | Complete blood counts (CBCs). | ||||
| •. | BM aspirate | ||||
| •. | Immunophenotyping was done using EPICS XL flow cytometer (Coulter Electronics, Hialeah, FL, USA). Panel included T-cell markers (CD1, CD3, CD4, CD5, CD7, CD8), B-cell markers (CD10, CD19, CD22, CD34, cytoplasmic u), and myeloid markers (MPO, CD13, CD33, HLA-DR). | ||||
Cytogenetic analysis
Pretreatment cytogenetic analyses of BM or peripheral blood (PB) were performed. Metaphases chromosomes were banded by G-banding technique and Karyotyped according to the International System for Human Cytogenetic Nomenclature. A minimum of 20 metaphases was required to be examined for a patient to be classified as having normal cytogenetic.
Leptin assay
DRG® Leptin (Sandwich) ELISA (EIA-2395) (R&D Systems, Minneapolis, MN, USA): The DRG Leptin ELISA kit is a solid-phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. The micro titer wells are coated with a monoclonal antibody directed towards a unique antigenic site on a leptin molecule. The intensity of color developed is proportional to the concentration of leptin in the sample. The concentration of the samples can be read directly from standard curve.
Adiponectin assay
R&D Quantikine Human Total Adiponectin/Acrp30 Immunoassay (DRP300) (R&D Systems). Quantitative sandwich enzyme immunoassay technique. The concentration of the samples can be read directly from standard curve.
SLDH ezyme assay
SLDH were determined on the day of admission or before start of chemotherapy was started in all acute leukemia patients. SLDH levels were determined using kits obtained from Biomerieux S.A. Marcy L' toile, France.
Statistical analysis
The statistical analysis of data was performed by using excel program and SPSS version 16 (statistical package for social science). Qualitative data were described in the form of numbers and percentages. Quantitative data were described in the form of mean (±) standard deviation (SD). Statistical analysis was performed by comparison between groups using chi-square test regarding qualitative data while quantitative non-parametric data comparison was performed using one-way analysis of variance and paired sample t-test. The probability of being by chance (P value) was calculated for all parameters (P is significant if < or =0.05 at confidence interval 95%).
Results
Hemoglobin and platelet counts were significantly lower and white cell count was significantly higher in acute leukemia patients as compared with controls (P = 0.00) ().
Table 1. Acute leukemia patient's characteristics
According to cytogenetic findings, the acute leukemia group was classified into three risky subgroups: favorable, intermediate, and unfavorable one. While the serum leptin levels were higher in the unfavorable group, followed by intermediate and the lowest levels in the favorable subgroup, the adiponectin levels were significantly higher in favorable subgroup followed by intermediate and lowest levels in the unfavorable group and the differences were statistically significant (P ≤ 0.00 for both) ().
Table 2. Adipocytokine levels (median, range) in different cytogenetically classified acute leukemia patients
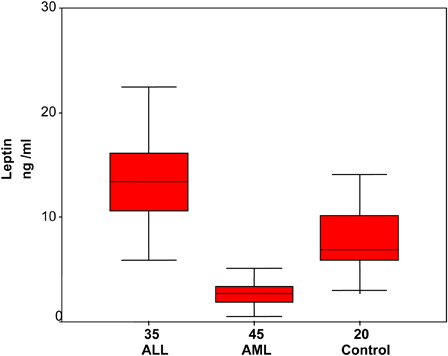
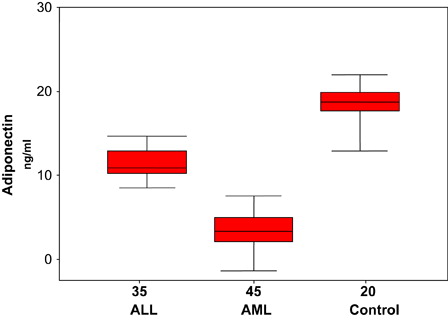
Serum leptin levels were significantly higher in acute lymphoblastic leukemia patients compared to controls (P = 0.01). On the other hand, serum leptin levels were significantly lower in acute myeloid leukemia (AML) as compared with controls (P = 0.00) (, ). Furthermore, serum adiponectin levels were significantly lower in ALL and AML patients compared to controls (P = 0.01, 0.00, respectively) (, ).
Table 3. Leptin and adiponectin levels in acute leukemia patients versus controls
Correlation studies regarding serum leptin levels; while there were positive significant correlation with BM blast cells percentage (r = 0.79, P < 0.001), blood total WBCs counts (r = 0.54, P < 0.01), sLDH (r 0.738, P < 0.001) in ALL group; there were significant negative correlations with BM blast cells (r 0.542, P < 0.01) and sLDH (r = −0.699, P < 0.001), and insignificant correlation with blood WBC counts (r 0.250, P > 0.05) in the AML group (Tables and ). Moreover, there were significant negative correlation between serum adiponectin levels and bone marrow blast cells and serum lactic dehydrogenase (LDH) in whole acute leukemia group (r −0.542, P < 0.01, r −0.699, P < 0.001, respectively) ().
Table 4. Correlation between serum adiponectin levels and BM blast cells, WBCs, sLDH in acute leukemia patients
Table 5. Correlation between serum leptin levels and BM blast cells, blood leucocytic counts, and sLDH in ALL patients
Table 6. Correlation between serum leptin levels and BM blast cells, WBCs, LDH in AML patients
No significant difference exists regarding BMI between acute leukemia patients when compared with normal controls (P > 0.05).
Discussion
Serum leptin levels were elevated in acute lymphoblastic leukemia patients as compared with normal controls (P = 0.01). The elevation in serum leptin levels were not related to BMI, as there was no significant difference between BMI in patients and controls. The source of this elevation might be lymphocytes and lymphoblast in addition to BM adipocytes. This finding is in agreement with that of Moschovi et al.Citation10 Pamuk et al.Citation11 explained an increase of serum leptin at acute lymphoblastic leukemia diagnosis as it is proinflammatory adipocytokines. Leptin immunoinflammatory mechanisms may contribute to cancer development, as this hormone displays proliferative and anti-apoptotic effects in a variety of cell types. Leukemia induces low-grade inflammation and serum lipids may stimulate leptin secretion. Sanchez-Margalet et al.Citation12 suggested that leptin alone and in combination with other cytokines have simulative effects on proliferation of leukemia cells as well as anti-apoptotic effects. Leptin has a trophic effect on monocytes, preventing apoptosis via the p42/44 MAPK pathway. Park et al.Citation13 mentioned that among leptin pleiotropic actions, leptin may function as an angiogenic factor.
Contrary to our finding, Fantuzzi and FaggioniCitation14 found reduction in serum leptin level in acute leukemia patients. They suggested that the cause rather seems to be multifactorial and related to the severe illness, altered energy balance, and disease complications. Bruserud et al.Citation15 found that although increased serum leptin and OB-R expression levels were found in many cancer types with related obesity, there are also reports stating the decrease in serum leptin levels in lymphoid and myeloid malignancies.
In the current study, serum leptin levels were significantly lower in AML as compared to normal controls (P = 0.00). Wallace et al.Citation16 explained the reduction in leptin concentrations in AML patients, although they had normal fat tissue as a defense mechanism from the body in order to keep appetite up to gain weight and to avoid weight loss. This may be due to disregulation in the feedback mechanisms developing in patients with hematologic malignancy. Tabe et al.Citation17 suggested that leptin has been shown to stimulate the proliferation of AML cells and to also have an anti-apoptotic effect. It increases the number of progenitor cells and spontaneous AML blast proliferation as well as AML blast release of IL-1beta, IL-6, tumor necrosis factor -alpha, and granulocyte-macrophage colony-stimulating factor.
In the present work, adiponectin levels were significantly lower in both AML and ALL patents as compared to healthy controls. The reduction in the adiponectin levels might be due to decrease in fat mass in the BM due to overcrowding the BM by blast cells; this is confirmed by the significant negative correlation between the BM blast cells counts and adiponectin levels in ALL and AML patients. This finding is in agreement with that reported in another types of cancer.Citation18 Previous studies demonstrated that circulating adiponectin is decreased in patients with breast, renal, prostate, colon or endometrial cancer.Citation4 These findings are in line with Yokota et al.Citation19 who hypothesize that adiponectin, a hormone that induces apoptosis, inhibits proliferation of myeloid, but not of lymphoid, cell lines. Adiponectin induces apoptosis in myelomonocytic progenitor cells in a dose-dependent manner, likely by down-regulating anti-apoptotic genes. Adiponectin serves as a negative regulator for myelomonocytic progenitor growth. In addition, adiponectin significantly inhibits functions of mature macrophages. Moreover, Brakenhielm et al.Citation20 suggested that tumor growth is angiogenesis-dependent, adiponectin alters neovascularization, and suppression of angiogenesis has been shown to inhibit tumor growth. Another potential mechanism by which adiponectin may exert its anti-proliferative, and thus anti-carcinogenic, its effects in regulating the bioavailability of certain growth factors.Citation21
Correlation studies revealed that there was a negative significant correlation between serum adiponectin levels and BM blast cells, as well as sLDH levels in acute leukemia groups. On the other hand, serum leptin levels was positively significantly correlated with BM blast cells, sLDH in the ALL group; and negatively significantly correlated with BM blast cells, sLDH in the AML group. These findings are in agreement with that reported by Molica et al.Citation22 They reported that adiponectin levels were inversely correlated with absolute PB lymphocytic counts, CD38-positive CLL cells and ZAP-70 in CLL patients.
In our study the acute leukemia was stratified into three subgroups (favorable, intermediate, and unfavorable) according to cytogenetic findings. Serum leptin levels were higher in the unfavorable group, followed by intermediate and the lowest levels were in the favorable subgroup. On the other hand, the adiponectin levels were significantly higher in favorable group followed by intermediate and lowest levels were in the unfavorable group and the differences were statistically significant. This finding points out for the prognostic value of serum adipocytokines levels in acute leukemia patients. Petridou et al.Citation23 have recently shown that circulating adiponectin is inversely associated with risk for AML. Moreover, Tonorezos et al.Citation24 found that higher leptin levels were associated with poor prognosis in acute lymphoblastic leukemia. Hino et al.Citation25 suggested that leptin has stimulative effects on proliferation of leukemia cells as well as anti-apoptotic effects. These findings suggest the possibility that leptin has poor prognosis in acute lymphoblastic leukemia.
In conclusion, adipocytokines may represent a new non-invasive biomarker in acute leukemia patients. Also the estimation of adiponectin and leptin serum levels at acute leukemia diagnosis could be considered as a prognostic marker which will be used in acute leukemia stratification.
References
- Bruserud Ø, Tore Gjertsen B. New strategies for the treatment of acute myelogenous leukemia: differentiation induction – present use and future possibilities. Stem Cells 2000;18:157–65.
- Attard-Montalto SP, Camacho-Hubner C, Cotterill AM. Changes in protein turnover, IGF-I and IGF binding proteins in children with cancer. Acta Paediatrica 1998;87:54–60.
- Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006;55:233–44.
- Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:S858–66.
- Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22.
- Hino M, Nakao T, Yamane T, Ohta K, Takubo T, Tatsumi N. Leptin receptor and leukemia. Leuk Lymphoma 2000;36:457–61.
- Konopleva M, Mikhail A, Estrov Z, Zhao S, Harris D, Sanchez Williams G, et al. Expression and function of leptin receptor isoforms in myeloid leukemia and myelodysplastic syndromes: proliferative and antiapoptotic activities. Blood 1999;93:1668–76.
- Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9.
- Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer 2006;94:1221–5.
- Moschovi M, Trimis G, Vounatsou M, Katsibardi K, Margeli A, Damianos A, et al. Serial plasma concentrations of adiponectin, leptin, and resistin during therapy in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:e8–13.
- Pamuk GE, Demir M, Harmandar F, Yesil Y, Turgut B, Vural O. Leptin and resistin levels in serum of patients with hematologic malignancies: correlation with clinical characteristics. Exp Oncol. 2006;28:241–4.
- Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11–19.
- Park HY, Kwon HM, Lim HJ. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95–102.
- Fantuzzi G, Faggioni R. mLeptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46.
- Bruserud Q, Huang TS, Glenjen N, Tore B, Foss B. Leptin in human acute myelogenous leukemia: studies of in vivo levels and in vitro effects on native functional leukemic blasts. Haematologica 2002;87:584–95.
- Wallace AM, Sattare N, Mc Millan DC. Effect of weight loss and the inflammatory response on leptin concentration in gastrointestinal cancer patients. Clin Cancer Res. 1998;4:2977–9.
- Tabe Y, Konopleva M, Igari J, Andreeff M. Spontaneous migration of acute promyelocytic leukemia cells beneath cultured bone marrow adipocytes with matched expression of the major histocompatibility complex. Rinsho Byori. 2004;52:642–8.
- Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA 2001;98:6390–5.
- Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 2000;96:1723–32.
- Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, et al. Adiponectin-induced antiangiogenesis andantitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA 2004;101:2476–81.
- Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–866.
- Molica S, Vitelli G, Cutrona G, Todoerti K, Mirabelli R, Digiesi G, et al. Prognostic relevance of serum levels and cellular expression of adiponectin in B-cell chronic lymphocytic leukemia. Intern J Hematol. 2008;88:374–80.
- Petridou E, Mantzoros CS, Dessypris N, Dikalioti SK, Trichopoulos D. Adiponectin in relation to childhood myeloblastic leukaemia. Br J Cancer 2006;94:156–60.
- Tonorezos ES, Vega GL, Sklar CA, Chou JF, Moskowitz CS, Mo Q, et al. Adipokines, body fatness, and insulin resistance among survivors of childhood leukemia. Pediatr Blood Cancer 2012;58:31–6.
- Hino M, Nakao T, Yamane T, Ohta K, Takubo T, Tatsumi N. Leptin receptor and leukemia. Leuk Lymphoma 2000;36:457–61.