Abstract
Purpose
The aim of this study is to assess the predictors of outcome in patients with relapsed or refractory Hodgkin's lymphoma (HL) receiving autologous stem-cell transplantation (ASCT)
Materials and methods
Fifty-two consecutive patients who received ASCT at the Stem Cell Transplantation Unit of Gazi University Hospital from February 2005 through June 2011 for relapsed or refractory HL were analysed retrospectively
Results
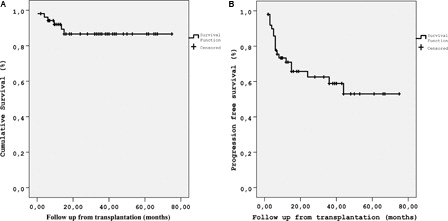
Fifty-one patients could be evaluated after transplantation, as one of the patients died in the early post-transplantation period. Complete remission was obtained in 36 (71%), partial remission in 9 (18%), stable disease in 4 (8%), and progressive disease in 2 (3%) patients. After a median follow-up of 22 (range, 0.5–75) months, 46 (88%) patients were alive. The probability of overall survival (OS), progression free survival (PFS) and transplantation related mortality at 5 years were 87, 53, and 2%, respectively. Chemosensitive relapse had a positive impact on both OS and PFS
Conclusion
ASCT remains to be the standard treatment of relapsed or refractory HL patients. Chemosensitive relapse is the most important prognostic factor determining the outcome of the ASCT.
Introduction
Introduction of anthracycline-based polychemotherapy provided the chance of cure for majority of the patients with Hodgkin lymphoma (HL) particularly when diagnosed in the early-stage of the disease.Citation1–Citation3 Whereas treatment failure occurs approximately in 10% of the patients with advanced disease and 30–40% of the patients relapse after achieving complete remission (CR).Citation2–Citation4 High-dose chemotherapy followed by autologous stem-cell transplantation (ASCT) has become the standard approach for the patients who fail to respond to induction chemotherapy (CT) or relapse after achieving CR and provides long-term disease-free survival.Citation2,Citation3
International prognostic score (IPS) defined by Hasenclever et al. has a widespread use all over the world for risk stratification of HL patients with advanced disease.Citation5,Citation6 However there are no sufficient data regarding the predictive value of IPS in relapsed or refractory patients who are candidates of ASCT. On the other hand some other factors such as chemosensitivity prior to ASCT,Citation7,Citation8 response to induction CT,Citation9 number of CT lines prior to ASCT,Citation7,Citation10,Citation11 achievement, and the duration of CR,Citation12,Citation13 presence of B symptoms,Citation9,Citation14 or extranodal involvementCitation15 at relapse have been described as prognostic factors for HL patients undergoing ASCT.
In this retrospective study, we aimed to analyse the outcome of a cohort of 52 relapsed or refractory HL patients who underwent ASCT and the impact of the previously described prognostic factors in predicting the outcome.
Patients and methods
Ninety-one patients with HL were treated at Gazi University Hospital between February 2005 and June 2011. Fifty-two of these 91 patients, with relapsed or primary refractory disease who were treated with ASCT at the same time period, were reviewed retrospectively. The relapse was histologically confirmed in 25 of the relapsed patients. The patients were evaluated with blood counts, erythrocyte sedimentation rate, lactate dehydrogenase, liver, and renal function tests. The patients were staged according to Ann Arbor classificationCitation16 at the time of diagnosis, relapse, and restaged prior to and 2–3 months after the ASCT. Physical examination, B symptoms, computed tomography scans or 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET-CT) scans, and bone marrow biopsy were included in the staging procedure. IPS at diagnosis was used to assess the prognosis.Citation5 Previous treatments and response to these treatments were recorded for each patient. Induction treatment was defined as the first treatment administered after diagnosis. Relapse was defined as the disease recurrence after achieving CR and primary refractory disease was defined as failure to achieve CR or partial response (PR) by induction CT and/or radiotherapy or as disease progression within 3 months of completing induction treatment. CR was defined as the absence of clinical and radiological evidence of disease. PR was defined as ≥%50 reduction in the greatest diameter in all sites of known disease and stable disease (SD) was defined as <%50 reduction in the greatest diameter in all sites of known disease. Progressive disease (PD) was defined as ≥%25 increase in size of known disease or development of new lesions. Any mass ≥10 cm was defined as bulky disease. Patients were defined to have chemosensitive disease when they achieved CR or PR after salvage treatment. Whereas chemoresistant disease was defined with responses less than PR after salvage treatment. Mobilisation protocols were as follows: granulocyte-colony stimulating factor (G-CSF minimum 10 µg/kg/day intravenous (IV) starting from day 1); cyclophosphamide (Cyc/G-CSF 4000 mg/m2 IV day 1, G-CSF minimum 10 µg/kg/day starting from day 2); Cyc/etoposide/G-CSF (Cyc 4000 mg/m2 IV on day 1, etoposide 600 mg/m2 on days 1–3, and G-CSF minimum 10 µg/kg/day starting from day 5); Gem/Vin/G-CSF (gemcitabine 1000 mg/m2 and vinorelbine 30 mg/m2 on days 1, 8, and 15, G-CSF minimum 10 µg/kg/day starting from day 17); ICE/G-CSF (etoposide 100 mg/m2/day on days 1–3; carboplatin AUC of 5 on day 1 to a maximum dose of 800 mg; ifosphamide 5 g/m2 with an equal dose of uromethexane on days 1–3, G-CSF minimum 10 µg/kg/day starting from day 5); IGEV (ifosphamide 2000 mg/m2 on days 1–4, uromethexane 2600 mg/m2 on days 1–4, gemcitabine 800 mg/m2 on days 1 and 4, vinorelbine 20 mg/m2 on day 1, prednisolone 100 mg on days 1–4, and G-CSF minimum 10 µg/kg/day starting from day 5). Stem cells were collected with the harvest procedure in one of the patients.
BEAM (BCNU 300 mg/m2 IV day −6, etoposide 800 mg/m2 IV days −5 to −2, cytarabine 1600 mg/m2 IV days −5 to −2, melphalan 140 mg/m2 IV day −2) was the conditioning regimen in all of the patients prior to ASCT.
Stem cells were infused to the patients 24 hours after the last dose of the conditioning CT. The day of stem cell infusion was considered as day 0. The day of neutrophil engraftment was defined as the first of the three consecutive days on which the absolute neutrophil count was ≥0.5 × 109/L. The day of platelet engraftment was defined as the first of the three consecutive days on which platelet count was ≥20 × 109/L without platelet transfusion. Primary graft failure was defined when myeloid engraftment was not reached until day 30. Antibacterial, antiviral, and antifungal prophylaxis was administered during pre- and post-transplantation period and supportive transfusions were given when indicated. Eight patients received radiotherapy after ASCT.
Statistical analysis
The SPSS 15 package programme was used in the analysis of statistical data. Data were presented as numbers and percentage or median and range, when appropriate. The chi-square test was used for the evaluation of categoric values and the Mann–Whitney U test for continuous values. Also, the Kaplan–Meier test with the log-rank analysis was used for the survival analysis. Cox-regression analysis was used for univariate and multivariate analysis. The variables were age, sex, presence of B symptoms at relapse prior to ASCT, chemosensitivity, number of CT lines prior to ASCT, response to induction CT, presence of a previous CR, and PET positivity prior to ASCT. Whereas the presence of B symptoms at relapse prior to the salvage CT was defined as presalvage risk factor, chemosensitivity to the salvage CT was accepted as post-salvage risk factor. All P values were two sided with the statistical significance at 0.05 alpha levels.
Results
Patient characteristics
Fifty-two relapsed or primary refractory HL patients who received ASCT were included in this retrospective study. Patient characteristics are shown in . The median age of the 12 female and 40 male patients included in the study was 31 (range, 16–60). Histological subtype was nodular sclerosis in 35 (67%), mixed cellular in 12 (23%), lymphocyte depleted in 3 (6%), lymphocyte predominant in 1 (2%), and unknown in 1 (2%) patient. Nineteen (37%) patients were at stage II, 11 (21%) patients were at stage III, 15 (29%) patients were at stage IV during diagnosis whereas the stage of the 7 (13%) patients were unknown. Majority of patients (92%) received ABVD as induction CT. Twenty-three (44%) had a history of radiotherapy, seven of whom had extended radiotherapy. Median number of CT lines prior to ASCT was two (range, 1–4) and this number was designated as the cut-off point. Twenty eight (54%) patients received ≤2 lines of CT prior to ASCT, whereas 24 (46%) patients received >2 lines of CT. Thirty-three (67%) patients had achieved a previous CR (data available in 49/52). The duration of CR was ≤12 months in 15 (45%) whereas 18 (55%) had a CR duration of >12 months. Response to the induction was CR in 25 (51%) patients, PR in 10 (20%), and primary refractory in 14 (29%), respectively (data available in 49/52). Thirty-five (67%) of the patients who underwent ASCT had relapsed disease. Pre-transplantation salvage regimens were Gem-Vin (n = 34), IGEV (n = 5), ICE (n = 6), ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) (n = 2), MiniBEAM (n = 1), DHAP (dexamethasone, cytosine arabinoside, and cisplatin) (n = 2), and ESHAP (etoposid, methylprednisolon, cytarabin, and cisplatin) (n = 2).
Table 1. Patient characteristics
Outcomes
The time interval between diagnosis and transplantation was median 839 (range, 210–9125) days. The median time to reach neutrophil and platelet engraftments were 11 days (range, 8–19) and 12 days (range, 8–30), respectively. Median number of CD34+ cells infused was 4.47 × 106/kg (range, 1.65–11.9 × 106/kg). Response to pre ASCT salvage CT was CR in 18 (35%), PR in 22 (42%), and refractory in 12 (23%) patients. Whereas 5 of the 12 primary refractory patients had SD, 7 of them had PD after the salvage CT administered prior to ASCT. Overall, 12 (23%) patients were chemoresistant and 40 (77%) patients were chemosensitive. Twenty-five (74%) of the patients receiving Gem-Vin, four (80%) of the patients receiving IGEV, four (67%) of the patients receiving ICE, and all the patients receiving the other regimens were chemosensitive.
Fifty-one patients could be evaluated after transplantation, because one of the patients died in the early post-transplantation period. CR was obtained in 36 (71%), PR in 9 (18%), SD in 4 (8%), and PD in 2 (3%) patients after transplantation. When 14 primary refractory patients – the ones who failed to respond to induction CT – were evaluated seperately; 8 (57%) patients were chemosensitive and 6 (43%) were chemoresistant to salvage CT implemented prior to ASCT. Seven of these (50%) achieved CR, three (22%) PR, two (14%) SD, and two (14%) had PD.
The subanalysis of the 12 patients who were refractory to salvage CT and undergone ASCT with refractory HL was as follows; two achieved CR, five achieved PR, and four had SD in the first post-transplantation evaluation and one had died early after transplantation before evaluation. After a median 22 months of follow-up three patients remained progression free, including the one in CR, and eight patients have progressed three of whom died.
Nineteen (38%) of the patients progressed after median 6 (range, 2–44) months. With a median follow-up of 22 (range, 0.5–75) months, 46 (88%) patients were alive. Whereas five (10%) patients died due to disease progression, only one (2%) transplant-related mortality (TRM) was observed. The probability of overall survival (OS), progression-free survival (PFS) and TRM of the cohort at 5 years were 87, 53, and 2%, respectively ().
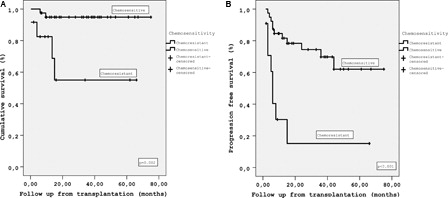
Probability of OS at 5 years was 95% in chemosensitive ASCT recipients whereas 55% in chemoresistant patients (P = 0.002), and the probability of PFS at 5 years was 62% in chemosensitive patients and 15% in chemoresistant patients (P < 0.001) (). When the chemosensitive patients were sub-categorized as the ones achieving CR and PR, the probability of OS at 5 years in patients with CR, PR were not different with 95%. The probability of PFS at 4 years in patients with CR, PR, and refractory disease were 81, 43, and 15%, respectively (P < 0.001). The difference in PFS between the patients in CR and refractory disease was significant (P < 0.001). However, the PFS was similar in patients with CR and PR, although the PFS was twice in patients who achieved CR (P = 0.113). With respect to the number of previous CT lines, the probability of OS at 5 years was 93% in patients with ≤2 lines of CT whereas 76% in patients with >2 lines (P = 0.181). The probability of PFS at 5 years was 63% in patients receiving ≤2 lines of CT and 41% in patients receiving >2 lines (P = 0.309). OS and PFS were also analysed with respect to the response to induction CT. The probability of OS at 5 years was 90% in patients who responded to induction CT and 73% in patients without a response (P = 0.177). Whereas the probability of PFS at 5 years was 62% in patients responding to induction CT and 25% in patients without a response (P = 0.003).
Figure 2. (A) OS of patients with chemosensitive and chemoresistant disease at ASCT (B) PFS of patients with chemosensitive and chemoresistant disease at ASCT.
Thirty-three patients had undergone pre-salvage and 51 patients had undergone pre-transplantation PET-CT evaluation. Thirty-two (63%) of 51 were PET positive, whereas 19 (37%) were negative. Fourteen of these 19 patients, had pre-salvage PET-CT scans and 13 of them were positive. The probability of OS at 5 years was 95% in PET negative patients and 80% in PET-positive patients (P = 0.204). The probability of PFS at 5 years was higher in PET-negative patients (77 vs. 37% P = 0.024).
Univariate and multivariate analysis
The prognostic variables at ASCT are summarized in . In univariate analysis only chemosensitive disease was found to have impact on both OS and PFS after ASCT (P < 0.05). In terms of PFS, chemosensitive disease, response to induction CT, a previous CR, and pre-transplantation PET were found to be significant variables in univariate analysis. When chemosensitive disease, response to induction CT, a previous CR, and pre-transplantation PET results were included in multivariate analysis, only chemosensitve disease was found to have an impact on PFS () (P = 0.028).
Table 2. Univariate analysis of prognostic variables
Table 3. Multivariate analysis of variables affecting PFS
Discussion
We report a cohort of relapsed or refractory HL patients who underwent ASCT in our stem cell transplantation unit. The probability of OS and PFS at 5 years was 87 and 53%, respectively. Although ASCT is the standard approach in relapsed and/or refractory HL patients, only two randomised control trials are available in the literature comparing ASCT with the standard salvage CT. In both studies PFS and OS were better in patients undergoing ASCT; nevertheless, a statistical significance was demonstrated for only PFS.Citation17,Citation18 The discrepancy between OS and PFS can be explained by the relatively smoldering course of HL even after multiple relapses. As relapsing patients might achieve multiple remissions with conventional treatment and a much longer follow-up is required to demonstrate a difference in OS. Similarly, the results of retrospective studies comparing patients undergoing ASCT with historic conventional treatment arm receiving salvage CT, ASCT favors a better OS rate however without statistical significance.Citation19,Citation20 The probability of OS at 5 years was reported to vary between 35 and 57% in patients undergoing ASCT in large retrospective studies.Citation7,Citation8,Citation11,Citation15,Citation19,Citation21,Citation22 OS rates have been preserved after adequate long-term follow-up of 10–15 years, with a probability rate of 48–54%.Citation9,Citation10,Citation22 With respect to PFS, the probability at 5 years ranged between 37 and 50%Citation7,Citation10,Citation11,Citation21,Citation22 and at 15 years was reported as 51%.Citation9 Our study demonstrates 87% probability of 5 year OS, which is superior to previous reports, and can be explained by improved transplant techniques with conditioning regimens free of total body irradiation (TBI), and better supportive care as well as novel and more effective salvage and induction regimens. Nevertheless, year of transplant was demonstrated to affect the non-relapse mortalityCitation8 and OS in previous studies.Citation7,Citation8,Citation10 Actually, previous reports by our group and others demonstrated that there are more effective salvage CT regimens including, ifosfamide, gemcitabine, and vinorelbineCitation23,Citation24 TBI-containing conditioning regimens were shown to increase non-relapse mortalityCitation8 and late TRM.Citation7 Unlike OS, PFS rate in our study was similar to the results in the previous studies, which should be interpreted as the results of ASCT should be further improved in HL.
Chemosensitivity is the most important post-salvage prognostic factor in predicting the outcome of relapsed or refractory HL patients undergoing ASCT.Citation3,Citation7,Citation8,Citation10,Citation11,Citation21 Sureda et al.Citation7,Citation8 demonstrated that sensitivity to salvage CT was the most important factor for both OS and PFS rates at 5 years in two large retrospective studies including 494 and 357 patients, respectively. Similar results were reported in smaller cohorts by Sweetenham et al.Citation21 and Majhail et al.Citation14 The results of the study by Sirohi et al.,Citation22 also showed that chemosensitivity affected both OS and PFS even at 10 years with more favorable results for patients entering transplantation in CR. Our results compare favorably with the above-mentioned reports. Chemosensitive disease has been shown to be an independent prognostic factor for PFS in a multivariate analysis which included response to induction, a history of CR, the number of prior lines of CT and pre-transplantation PET positivity as variables. Chemosensitive disease was also demonstrated to be an important prognostic factor for OS, in univariate analysis. Further categorizing chemosensitive patients according to the depth of response, OS at 5 years was similar in patients achieving CR or not as long as they are chemosensitive. A longer follow-up, though might be required to demonstrate the impact of CR on OS. It should also be noted that both of the randomized trials conducted in relapsed HL patients to test the impact of ASCT does not include chemorefractory patients.Citation17,Citation18 Despite the limitation of our study because of its retrospective nature, relatively small sample size, and short follow-up, we should mention that 25% of the patients refractory to salvage regimens and entered to transplant with active disease remain progression free and 75% are alive. Another important result of this study which could not be ignored is increased PFS in patients achieving CR compared with patients with PR, though not significant statistically (81 vs. 43%).
Determining pre-salvage and post-salvage risk factors such as chemosensitivity might lead to risk-adapted strategies including modifying salvage and conditioning regimens. Pre- or post-transplantation maintenance or consolidation treatment with novel agents such as brentuximabCitation25 and histone deacetylase inhibitorsCitation26 and/or involved field radiotherapyCitation4 could be considered in selected patients who fail to achieve CR. Results of the AETHERA study (ClinicalTrials gov identifier: NCT01100502), a multicenter phase 3 study evaluating the efficacy of brentuximab vedotin in the treatment of post-transplantation residual and high-risk disease in patients who undergo ASCT with the diagnosis of HD, will provide significant contribution to patients with persistent disease. On the other hand, the best PFS in our series is achieved in patients who achieve CR prior to transplantation, which suggests that novel treatments might be challenged prior to transplantation in patients who fail to achieve CR. It should be noted that the similar OS in the chemosensitive patients independent from achieving CR might be misleading as being disease free is known to be the strongest indicator of OS. Much longer follow-up than ours is required to demonstrate the impact of being disease free on OS. Furthermore, at least a subset of patients with chemorefractory disease might in fact be radiosensitive. Induction failure in HL is an extremely poor prognostic condition resulting in OS rates of 8–26% when further treated with conventional CT.Citation27–Citation29 In a large trial from EBMTCitation30 including patients who not only failed to respond to induction CT but also to second line CT, OS and PFS rates at 5 years after ASCT were 36 and 32%, respectively. The study by Constans et al.Citation31 demonstrated even worse OS and time to treatment failure (26 and 15%, respectively) at 5 years in patients with induction failure. Smith et al.Citation15 on the other hand reported better results with an OS of 55% and EFS of 45% at 6 years in 214 patients with induction failure. However, early-relapsed (within 1 year) patients were also included in the study group in that study. A study with a long-term follow-up from Vancouver which includes 100 HL patients, OS and PFS at 15 years were better in patients who responded to induction CT (67 vs. 39% and 62 vs. 39%, respectively).Citation9 In an other study with long-term follow-up of 10 years, patients with primary refractory and relapsed disease had similar OS and PFS (48 vs. 47% and 45 vs. 45%).Citation14 The discrepancy in these studies may arise from their heterogenous patient population with different induction and salvage therapies, different high-dose conditioning regimens, different time period of transplantation. The probability of 5-year OS and PFS in our patients refractory to induction CT were 73 and 25%, respectively.
Another important point which should be addressed is the impact of the number of previous CT lines on the outcome of ASCT. OS and PFS rates in our cohort were superior in patients receiving ≤2 lines of CT, though without statistical significance. Sureda et al.Citation7 in a series of 494 patients showed that the number of previous CT lines had an impact on both OS and PFS. Whereas Moskowitz et al.Citation10 and Chopra et al.Citation11 demonstrated that the number of previous regimens had an impact only on PFS. Requirement of more CT lines seems to be an indicator of biologic behaviour of the tumor and the smaller sample size in our study might explain the lack of statistical significance. There have been additional pre-salvage risk factors defined in previous studies such as the presence of bulky disease,Citation15 extranodal diseas,Citation9,Citation14,Citation15 and B symptoms.Citation9,Citation14 We could not demonstrate any prognostic significance of B symptoms. And other risk factors could not be evaluated due to smaller sample size.
The absence of secondary malignancies such as acute myeloid leukemia, myelodysplastic syndrome, NHL, and lung cancer in our cohort, unlike the previous reportsCitation32,Citation33 could well be explained by the relatively short follow-up period.
Conclusion
In conclusion, ASCT remains to be the treatment of choice in relapsed and/or refractory HL patients. The OS rates have improved in the recent years and suggest the impact of improved supportive treatment and more effective salvage treatments. However, PFS requires further improvement. Chemosensitive disease, response to induction CT, presence of B symptoms seems to be important prognostic factors. Chemosensitive disease results in superior OS and PFS in relapsed/refractory HL patients. Risk-adapted chemo/radiotherapy salvage and consolidation treatments, tandem autologous and/or allogeneic stem cell transplantation might further improve the results in patients with chemorefractory disease where the prognosis remains to be poor. Further improving the depth of response prior to transplantation with novel agents seems to be a promising research topic of the future randomized trials as the best results post-transplantation are achieved in complete responders.
References
- Armitage JO. Early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:653–62.
- Küppers R, Yaholom J, Josting A. Advance in biology, diagnostics, and treatment of Hodgkin's disease. Biol Blood Marrow Transplant. 2006;12:66–76.
- Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011;117:4208–17.
- Kuruvilla J. Standard therapy of advanced Hodgkin lymphoma. Hematol Am Soc Hematol Educ Program. 2009;497–506.
- Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International prognostic factors project on advanced Hodgkin's disease. N Engl J Med. 1998;339:1506–14.
- Bierman PJ, Lynch JC, Bociek RG, et al. The international prognostic factors project score for advanced Hodgkin's disease is useful for predicting outcome of autologous hematopoietic stem cell transplantation. Ann Oncol. 2002;13:1370–77
- Sureda A, Arranz R, Iriondo A, et al. Autologous stem-cell transplantation for Hodgkin's disease: results and prognostic factors in 494 patients from the Grupo Español de Linfomas/Transplante Autólogo de Médula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19:1395–1404.
- Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16:625–33.
- Lavoie JC, Connors JM, Phillips GL, et al. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood 2005;106:1473–78.
- Moskowitz CH, Kewalramani T, Nimer SD, Gonzalez M, Zelenetz AD, Yahalom J. Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory Hodgkin's disease. Br J Haematol. 2004;124:645–52.
- Chopra R, McMillan AK, Linch DC, et al. The place of high-dose BEAM therapy and autologous bone marrow transplantation in poor-risk Hodgkin's disease. A single-center eight-year study of 155 patients. Blood. 1993;81:1137–45.
- Moskowitz CH, Nimer SD, Zelenetz AD, et al. A two-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–23.
- Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. J Clin Oncol. 2002;20:221–30.
- Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–72.
- Smith SD, Moskowitz CH, Dean R, et al. Autologous stem cell transplant for early relapsed/refractory Hodgkin lymphoma: results from two transplant centres. Br J Haematol. 2011;153:358–63.
- Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–6.
- Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4.
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–71.
- André M, Henry-Amar M, Pico JL, et al. Comparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin's disease induction failure: a case-control study. Société Francaise de Greffe de Moelle. J Clin Oncol. 1999;17:222–9.
- Yuen AR, Rosenberg SA, Hoppe RT, Halpern JD, Horning SJ. Comparison between conventional salvage therapy and high-dose therapy with autografting for recurrent or refractory Hodgkin's disease. Blood. 1997;89:814–22.
- Sweetenham JW, Taghipour G, Milligan D, et al. High-dose therapy and autologous stem cell rescue for patients with Hodgkin's disease in first relapse after chemotherapy: results from the EBMT. Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1997;20:745–52.
- Sirohi B, Cunningham D, Powles R, et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma. Ann Oncol. 2008;19:1312–9.
- Suyani E, Sucak GT, Akı ŞZ, Yeğin ZA, Özkurt ZN, Yağcı M. Gemcitabine and vinorelbine combination is effective in both as a salvage and mobilization regimen in relapsed or refractory Hodgkin lymphoma prior to ASCT. Ann Hematol. 2011;90:685–91.
- Santoro A, Magagnoli M, Spina M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin's lymphoma. Haematologica. 2007;92:35–41.
- Gualberto A. Brentuximab Vedotin (SGN-35), an antibody-drug conjugate for the treatment of CD30-positive malignancies. Expert Opin Investig Drugs. 2012;21:205–16.
- Buglio D, Younes A. Histone deacetylase inhibitors in Hodgkin lymphoma. Invest New Drugs. 2010;28:21–7.
- Bonfante V, Santoro A, Viviani S, et al. Outcome of patients with Hodgkin's disease failing after primary MOPP-ABVD. Clin Oncol. 1997;15:528–34.
- Radman I, Basić N, Labar B, et al. Long-term results of conventional-dose salvage chemotherapy in patients with refractory and relapsed Hodgkin's disease (Croatian experience). Ann Oncol. 2002;13:1650–55.
- Josting A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood. 2000;96:1280–6.
- Sweetenham JW, Carella AM, Taghipour G, et al. High-dose therapy and autologous stem-cell transplantation for adult patients with Hodgkin's disease who do not enter remission after induction chemotherapy: results in 175 patients reported to the European Group for Blood and Marrow Transplantation. Lymphoma Working Party. J Clin Oncol. 1999;17:3101–9.
- Constans M, Sureda A, Terol MJ, et al. Autologous stem cell transplantation for primary refractory Hodgkin's disease: results and clinical variables affecting outcome. Ann Oncol. 2003;14:745–51.
- Tesch H, Sieber M, Diehl V. German Hodgkin Study Group. Treatment of advanced stage Hodgkin's disease. Oncology. 2001;60:101–9.
- Goodman KA, Riedel E, Serrano V, Gulati S, Moskowitz CH, Yahalom J. Long-term effects of high-dose chemotherapy and radiation for relapsed and refractory Hodgkin's lymphoma. J Clin Oncol. 2008;26:5240–7.