Abstract
A mixing test is useful for distinguishing between coagulation factor deficiency and the presence of inhibitor as the cause of coagulopathy. However, we experienced a patient with acquired factor V (FV) inhibitor whose mixing test showed a coagulation factor deficiency pattern. A 65-year-old man with a tendency for bleeding was referred to our center. The laboratory data showed remarkable prolongation of prothrombin time and activated partial thromboplastin time (APTT). FV activity was less than 3%. A mixing test showed a coagulation factor deficiency pattern. However, neither the tendency for bleeding nor the coagulation tests were corrected by transfusion of fresh frozen plasma. A few days later, a positive test for FV inhibitor of 3 Bethesda units was obtained. Therefore, we started prednisolone and plasma exchange, and the coagulation test results normalized after 6 weeks. Although an incubation period is generally not considered necessary in a mixing test for FV inhibitor, we repeated mixing tests with various incubation periods and confirmed an incubation period-dependent prolongation of the APTT. Therefore, a mixing test with an incubation period is recommended for the detection of FV inhibitor, since a mixing test without an incubation period may show a coagulation factor deficiency pattern when the titer of FV inhibitor is low.
Introduction
Acquired factor V (FV) inhibitor is a rare condition that is associated with clinical symptoms ranging from asymptomatic laboratory abnormalities to life-threatening bleeding.Citation1 Most patients with FV inhibitor have underlying diseases or risk factors, including surgical procedures, exposure to topical bovine thrombin,Citation2,Citation3 antibiotic administration,Citation4 blood transfusions, cancers,Citation5 and autoimmune disorders.Citation6,Citation7 A prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT) are usually seen as a laboratory coagulation profile in this condition.Citation8 A mixing test is useful for distinguishing between coagulation factor deficiency and the presence of inhibitor as the cause of coagulopathy. In a mixing test, the patient's plasma is mixed with normal pooled plasma, and coagulation tests that include PT and APTT are repeated. Correction of any abnormalities in the coagulation test generally implies a coagulation factor deficiency. On the other hand, a failure to correct abnormalities in the coagulation test suggests the presence of an inhibitor.Citation9
We report here a patient who developed a tendency for severe bleeding with a mixing test result that showed a coagulation factor deficiency pattern. However, the presence of FV inhibitor was confirmed by the Bethesda method.Citation1 The coagulopathy was not corrected by transfusion of fresh frozen plasma (FFP) and platelet concentrates (PC). Therefore, there was a discrepancy between the mixing test results and the clinical and laboratory data.
Case report
A 65-year-old man with a medical history of hypertension and gout developed bloody stool, gross hematuria, and nasal bleeding. He visited a local hospital because the bleeding symptoms persisted for 2 weeks, and was shown to have marked abnormalities on coagulation studies: PT 142.8 seconds and APTT unmeasurable prolongation. He was immediately admitted and treated with FFP and vitamin K, but there was no improvement. He was referred to our medical center for the investigation of severe coagulopathy.
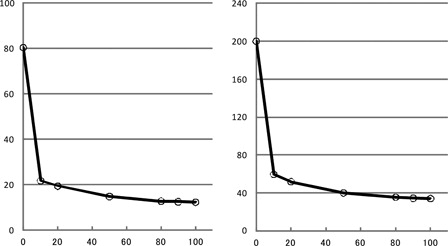
On admission, gastrointestinal bleeding was observed. A physical examination revealed several ecchymoses on his extremities. Complete blood count showed mild anemia of hemoglobin 10.6 g/dl. The platelet count was 342 × 109/l and the bleeding time was 2.5 minutes (normal: 2–5 minutes). Liver and renal function tests were within the normal limits. Marked abnormalities were observed in coagulation studies: PT 55.4 seconds (normal: 10–13 seconds), APTT >200 seconds (normal: 25–40 seconds), fibrinogen 362 mg/dl (normal: 150–380 mg/dl), fibrin/fibrinogen degradation product 1.2 µg/ml (normal: <5 µg/ml), D-dimer <1.0 µg/ml (normal: <1.0 µg/ml), and antithrombin-III 95.0% (normal: 70–120%). In a mixing test, both PT and APTT were corrected by mixing with normal plasma, which implied a coagulation factor deficiency (). A coagulation factor assay showed a decrease in FV activity of <3% (normal: 75–135%). Other coagulation factor activities were within normal limits: factor II activity 80% (normal: 75–135%), factor VIII (FVIII) activity 127% (normal: 60–150%), factor IX activity 96% (normal: 70–130%), and factor X activity 87% (normal: 70–130%). Antiphospholipid antibodies were not detected.
Figure 1. Results of a mixing test. Both the prothrombin time and activated partial thromboplastin time (APTT) were corrected by a small amount of normal plasma, which implied coagulation factor deficiency.
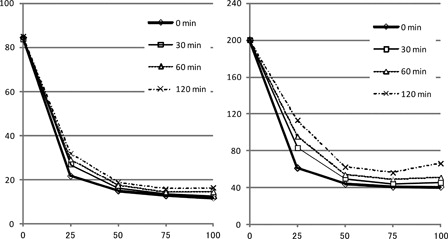
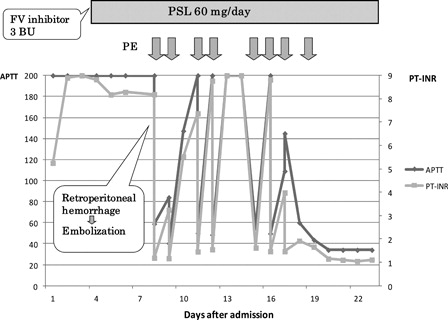
The clinical course is summarized in . The patient was initially treated with daily transfusion of FFP and PC based on the results of a mixing study. However, neither PT nor APTT was corrected even immediately after the transfusion of FFP. Four days after admission, an FV inhibitor test was positive at 3 Bethesda units (BU). Therefore, we started prednisolone (PSL) at 1 mg/kg to suppress inhibitor production. Since there was a discrepancy between the results of the mixing test and the clinical data, we performed the mixing test again with various incubation periods (). Although the test showed a coagulation deficiency pattern similar to the previous test, the coagulation time tended to increase with an increase in the incubation period at the same mixing ratio. One week after admission, the patient suddenly developed severe pain in his left groin. Enhanced computed tomography (CT) showed massive hematoma of his left iliopsoas muscle with persistent active bleeding. We decided to perform plasma exchange (PE) for the immediate elimination of inhibitor. Immediately after PE, PT and APTT were corrected to the normal levels. Next, intravascular embolization of the left deep circumflex iliac artery and left lumbar artery through the right femoral artery was successfully performed by using Histoacryl® (B. Braum Aesclup, Tokyo, Japan) blue as an obstructing material. However, the next morning, PT and APTT were again markedly prolonged. Although PE provided only a temporary benefit, we decided to continue PE once daily or every other day until the effect of immunosuppressive therapy became apparent. Approximately 2 weeks after starting PSL, improvement of the coagulation time was observed. PE was stopped after a total of eight iterations. We continued PSL at 1 mg/kg for 4 weeks, and then started to taper gradually. FV activity was 114 and 146% at 6 and 12 weeks after starting PSL, respectively. FV inhibitor was not detected at these time points. As the underlying disease of acquired FV inhibitor, rectal cancer was suspected based on CT findings. After the coagulation abnormalities were corrected, he underwent colonoscopic examination, which led to a diagnosis of rectal cancer. Elective surgical resection was performed successfully. Prednisolone was stopped 5 months after its initiation, and there has been no relapse of coagulopathy thus far.
Figure 2. Clinical course after admission. Prothrombin time and activated partial thromboplastin time were extremely prolonged on admission. Prednisolone at 1 mg/kg was started 5 days after admission when tests were positive for FV inhibitor. At 8 days after admission, he developed massive retroperitoneal hemorrhage, which was successfully controlled by urgent plasma exchange (PE) and intravascular embolization. PE was repeated daily or every other day until coagulopathy was improved.
Discussion
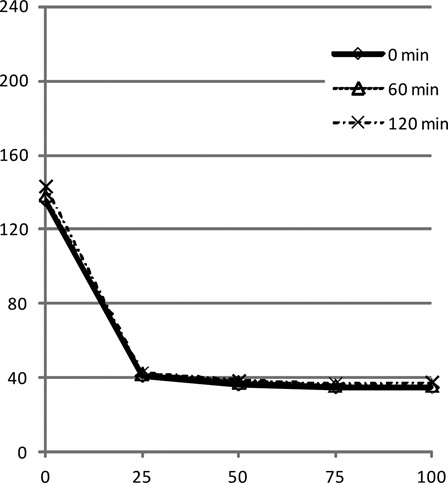
The clinical course and the inhibitor test of the current patient showed the presence of FV inhibitor, but the mixing test showed a coagulation factor deficiency pattern. As a possible explanation for this discrepancy, we considered that the incubation period after mixing with normal plasma might be insufficient to inactivate the coagulation factor in normal plasma. In general, FV inhibitor has been considered to bind and neutralize FV instantly, and therefore an incubation period is not routinely incorporated in the mixing test for FV inhibitor, in contrast to that for FVIII inhibitor.Citation10,Citation11 Accordingly, we initially performed the mixing test without an incubation period (). However, the result was not consistent with the clinical course. Therefore, we repeated the mixing test with various incubation periods, such as 30, 60, and 120 minutes (). The curve still showed a deficiency pattern with various incubation periods, but PT and APTT tended to increase with an increase in the incubation period. To confirm that the prolongation of PT and APTT was due to the binding of FV inhibitor and FV, we carried out a mixing test using FV-deficient plasma and normal pooled plasma (). The different incubation periods did not affect APTT in the mixing tests with FV-deficient plasma and normal plasma. Therefore, the incubation period-dependent prolongation of APTT in the current patient resulted from the presence of FV inhibitor in his plasma. These findings are consistent with the clinical course. In this patient, PT and APTT improved immediately after PE, but then increased again several hours later. This temporary improvement and later worsening might, at least partly, be the result of slow binding between FV and FV inhibitors.
Figure 4. Results of a mixing test with FV-deficient plasma and normal plasma. A difference in incubation period did not affect the results.
Two recent reports have suggested that the incubation period might affect the results of a mixing test for FV inhibitor ().Citation12,Citation13 In Gartrell's report, while APTT was not corrected by mixture of the patient's plasma and normal plasma, APTT in the mixing test was increased from 104.7 seconds to 119.8 seconds with 1 hour of incubation. Lipshitz et al. also reported that APTT in a mixing test with a 1-hour incubation period was longer than that without an incubation period, but the APTT was corrected to nearly the normal value. The BU of FV inhibitor in these patients were 17 and 6, respectively. In the current patient with a FV inhibitor of 3 BU, APTT was normalized by a 1:1 mixture with normal plasma without incubation, but was prolonged after incubation for 30 minutes or longer. If we consider these findings together, a mixing test without incubation may overlook the presence of FV inhibitor at a low level (i.e. <6 BU), and therefore, we should routinely compare the APTT values of mixing tests with and without an incubation period. In the absence of FV inhibitor, there should be no change in the APTT values with various incubation periods ().
Table 1. Effect of an incubation period in a mixing test for Factor V inhibitor
In general, the treatment of acquired FV inhibitor is based on supplying the coagulation factor and eradication of the autoantibody. To supply FV, FFP, and PC, which contains platelet-derived FV in the alpha granules, are administered, but in most cases their effect is insufficient. Therefore, immunosuppressive therapy to suppress autoantibodies is essential. Corticosteroids alone or in association with cyclophosphamide or other immunosuppressants have been used successfully to suppress inhibitor production. The overall probability of disappearance of the inhibitor is 88%, and the median time to disappearance is 9.7 weeks.Citation1 The current patient, who was treated with PSL alone as an immunosuppressive regimen and PE, achieved normal coagulation tests within 2 weeks and the disappearance of FV inhibitor was confirmed at 6 weeks. Although the effect of PE might be transient, PE can be beneficial in life-threatening hemorrhage by rapidly reducing the inhibitor titer and refilling coagulation factor.Citation14 We also performed intravascular embolization for the treatment of life-threatening retroperitoneal arterial bleeding. For intravascular embolization, we used Histoacryl® blue as an obstructing material. Although most obstructing materials work with coagulation factors, Histoacryl® blue can embolize a vessel in the absence of coagulation factors, and thus can be used in the setting of coagulation factor deficiency.
With regard to the possible causes for the production of inhibitors, the patient had no history of bovine thrombin and antibiotic use before the bleeding episode. As mentioned in the previous reports,Citation7 we considered that the inhibitor was produced associated with rectal cancer, as coagulopathy and bleeding symptom did not relapse after the resection of tumor.
In summary, when the titer of FV inhibitor is low, the results of a mixing test without an incubation period may show a coagulation factor deficiency pattern even in the presence of inhibitor. Therefore, a mixing test with an incubation period is recommended when the presence of FV inhibitor is suspected.
Acknowledgement
The authors thank Tatsuro Watano for his technical assistance and advice.
References
- Knobl P, Lechner K. Acquired factor V inhibitors. Baillieres Clin Haematol. 1998;11:305–18.
- Zehnder JL, Leung LL. Development of antibodies to thrombin and factor V with recurrent bleeding in a patient exposed to topical bovine thrombin. Blood 1990;76:2011–6.
- Streiff MB, Ness PM. Acquired FV inhibitors: a needless iatrogenic complication of bovine thrombin exposure. Transfusion 2002;42:18–26.
- Stenbjerg S, Husted S, Mygind K. A circulating factor V inhibitor: possible side effect of treatment with streptomycin. Scand J Haematol. 1975;14:280–5.
- Endo H, Kawauchi K, Tomimatsu M, Iga D, Ogasawara T, Yasuyama M, et al. Acquired factor V inhibitor responsive to corticosteroids in a patient with double cancers. Intern Med. 2007;46:621–5.
- Koyama T, Saito T, Kusano T, Hirosawa S. Factor V inhibitor associated with Sjogren's syndrome. Br J Haematol. 1995;89:893–6.
- Franchini M, Lippi G. Acquired factor V inhibitors: a systematic review. J Thromb Thrombolysis 2011;31:449–57.
- Ang AL, Kuperan P, Ng CH, Ng HJ. Acquired factor V inhibitor. A problem-based systematic review. Thromb Haemost. 2009;101:852–9.
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82:864–73.
- Ortel TL, Quinn-Allen MA, Charles LA, Devore-Carter D, Kane WH. Characterization of an acquired inhibitor to coagulation factor V. Antibody binding to the second C-type domain of factor V inhibits the binding of factor V to phosphatidylserine and neutralizes procoagulant activity. J Clin Invest. 1992;90:2340–7.
- Coots MC, Muhleman AF, Glueck HI. Hemorrhagic death associated with a high titer factor V inhibitor. Am J Hematol. 1978;4:193–206.
- Gartrell B. Acquired factor V inhibitor complicating warfarin therapy. Am J Hematol. 2011;86:710–2.
- Lipshitz J, Chelliah T, Aledort L. A case of factor V inhibitor with complete correction of the PT and aPTT upon mixing. Am J Hematol. 2012;87:313–5.
- Fu YX, Kaufman R, Rudolph AE, Collum SE, Blinder MA. Multimodality therapy of an acquired factor V inhibitor. Am J Hematol. 1996;51:315–8.