Abstract
Objective
This study sought to examine implantation and implantation kinetics in double umbilical cord blood transplantation (DUCBT).
Methods
Twenty-nine patients who underwent a two-unit unrelated donor cord blood transplantation were included in this study. After transplantation, hematopoietic chimerism of the peripheral blood was evaluated based on the results of short tandem repeat polymerase chain reaction. Using these results, we were able to judge whether the transplanted cells implanted, determine which donor's cells implanted, and further examine the kinetics of implantation in DUCBT. The numbers of total nucleated cells (TNCs), CD34+ cells, colony forming units (CFUs), colony forming unit-granulocytes and macrophages (CFU-GMs), and CD3+ cells were compared between the dominant units and the non-dominant units in an attempt to understand the discipline and implantation kinetics of DUCBT.
Results
Neither the TNC counts nor the counts of CD34+ cells, CFU, CFU-GM, or CD3+ cells were significantly different between the dominant units and the non-dominant units (P values of 0.584, 0.322, 0.842, 0.534, and 0.082, respectively).
Conclusions
We were able to determine the engraftment status at 14 days after DUCBT, although the implantation kinetics of DUCBT remain uncharacterized and require further research.
Research background
Since the first report of a successful umbilical cord blood transplantation (DUCBT) in patients with Fanconi anemia in 19881, there has been great interest in the use of cord blood as an alternative stem cell source with which to treat cancer and genetic diseasesCitation2–Citation7. Another use of UCBT has been to treat malignant hematologic diseases. Although the advantages of UCBT include its ready availability, the lack of harm to the donor, and a lower incidence and severity of graft-versus-host disease (GVHD) following transplantation, UCBT is restricted in its clinical applications, especially for adults and high-weight children due to the low cell number in umbilical cord bloodCitation8. Currently, DUCBT is regarded as a method that could resolve the limitation of the low cell number of umbilical cord bloodCitation9. The primary concern related to UCBT is the potential for implantation delay and high rates of early transplantation rejection. The median time for absolute neutrophil count (ANC) and blood platelet has commonly been used to determine hematopoietic recovery; however, given that this is an indirect method of judging the treatment, early opportunities to cure the underlying disease can be lost. To perform successful interventions and life-saving measurements in the early days following the procedure, it is very important to research DUCBT engraftment and establish another standard that could evaluate engraftment status accurately during the early period following engraftment. After infusing two units of umbilical cord blood, we detected the graft chimerism status using short tandem repeat polymerase chain reaction (STR-PCR) for the peripheral blood or bone marrow of the patient. STR-PCR dual amplification technology is a method for the detection of chimerism, and it has higherCitation10 specificity and sensitivity and is one of the most sensitive techniques for recognizing graft implantation status following hematopoietic stem cell transplantationCitation11,Citation12. HJ KangCitation13 and Claudio GCitation14 reported that this method is also applicable for chimerism detection following DUCBT; we therefore used STR-PCR to detect graft chimerism status changes following DUBCT to examine DUCBT implantation and to provide a reliable basis by which the effectiveness of the treatment can be made during the early period post-transplantation.
Given that the neither the characteristics nor the kinetics of dominant engrafting umbilical cord blood are well understood, most of the observations that are made regarding the dominant unit following DUCBT remain explained. BarkerCitation6 and others reported in 26 patients who received DUCBT that there was no connection between the dominant unit and its total nucleated cell (TNC) or CD34+ cell count, donor and acceptor gender consistency, the units ABO blood type, the degree of human leukocyte antigen (HLA) match, the infusion sequence of the units, etc. Recently, Sharon et al.Citation15 reported that the dominant implanting umbilical cord blood had a high CD3+ cell count (P = 0.04). Furthermore, there is also no consensus in the literature with respect to DUCBT implantation kinetics. Thus, this article will discuss DUCBT engraftment kinetics using the statistical analysis of DUCBT clinical data at a single transplantation center.
Research object and method
Case source
We selected 29 hematonosis patients who received DUCBT in the hematology department of the Provincial Hospital of Anhui Medical University between November 2005 and May 2011. The patient sample included 22 male and 7 female, and the median age was 23 years (range: 10–48 years). The median weight was 63 kg (range: 31–80 kg). The disease diagnoses included the following: chronic myeloid leukemia (CML), 11 cases; acute myeloid leukemia (AML), 7 cases; acute lymphoblastic leukemia, 9 cases; acute hybrid leukemia, 1 case; and myelodysplastic syndrome, 1 case. There were nine high-risk patients, five refractory patients, and five patients with progressing disease. The HLA match statistics were as follows: 1 case of 6, 6/6; 8 cases of 6, 5/6; 9 cases of 5, 5/6; 7 cases of 5, 4/6; 3 cases of 4, 6/6; and 1 case of 4, 4/6.
Implantation plan
The pre-treatment plans consisted of the following: total body irradiation (TBI) + cytosine arabinoside + cyclophosphamide (CY) ± bis-chloroethyltrosourea (BCNU) in 19 cases; busulfan (BU) + CY ± BCNU ± ATG in 4 cases; and fludara + BU + anti-thymocyte globulin (TBI + ATG) in 6 cases. The GVHD prevention plan consisted of cyclosporin A + mycophenolate mofetil. The median TNC count of the input double umbilical cord blood was 4.70 (3.26–7.70) × 107/kg (recipient's weight), and the median CD34+ cell count was 2.41 (0.61–5.23) × 105/kg (recipient's weight) (see ).
Table 1. DUCBT patients information list
Experimentation method and sample collection
We collected 2 ml of peripheral blood from patients in an ethylenediaminetetraacetic acid anticoagulant tube prior to the DUCBT and from the blood that remained in the umbilical cord blood transfusion bag. On the 7th, 14th, and 21st day post-DUCBT, we collected approximately 2 ml of peripheral blood into the same types of tubes from the treated patients, and these samples were sent, along with the peripheral blood samples that were taken prior to implantation, for STR-PCR analysis to Hefei Municipal Criminal Police Detachment of Anhui province. On the 30th day, we extracted bone marrow from the patients to perform the STR-PCR analysis; specialized staff members were responsible for the submission of the samples and the statistical results of the STR-PCR analyses.
Based on the STR-PCR results, we judged the influence of the implant umbilical cord blood on the recipients and analyzed the characteristics of the implanted umbilical cord blood and the engraftment kinetics of DUCBT.
Judging criteria and statistical methods
A variety of chimerism statuses can be detected via STR-PCR detection. A 100% contribution of donor is referred to as 100% donor type (full donor chimerism with no recipient ingredient, FDC). No chimerism is referred to as 100% recipient type (no donor chimera, NDC). If the result indicates that the recipients carry recipients ingredients and umbilical cord blood from one or two donors, the situation is referred to as mixed chimerism (MC). Most DUCBT patients achieve 100% one umbilical cord blood advantaged implantation and fewer exhibit significant contributions from both donors. Therefore, for the statistical analysis, we regarded FDC as a successful implantation, MC and NDC as a lack of implantation. In one case, both implants engrafted long-term (double chimerism with no recipient ingredient), which was confirmed by monitoring the results, indicating that chimeric implantation occurred for both of the units. Therefore, the statistical analysis was performed based on the chimerism status at different time points following implantation. To analyze the chimerism and implant status on the 7th, 14th, 21st, and 30th days after implantation, we used a consistency test for the statistical analysis on the basis of previous research and analysis of DUCBT implantation. In addition, statistical analysis was performed on the samples of umbilical cord blood that implanted and those that did not. For this analysis, the following metrics were determined and analyzed using the Wilcoxon paired test: TNC count following resuscitation, CD34+ cell number following resuscitation, colony forming unit (CFU) and colony forming unit-granulocytes and macrophage (CFU-GM) number prior to freeze, and CD3+ cell count following resuscitation. These analyses were performed to characterize the implanted umbilical cord blood and to study DUCBT engraftment kinetics. SPSS 17.0 statistical analysis software was used, and P < 0.05 indicates statistical significance.
Results
Engraftment information following DUCBT
In 29 cases of DUCBT, 24 patients achieved implantation (100% FDC), including 23 cases of single-donor implantation and 1 case of proportional balanced chimeric implantation with no recipient ingredient. There were two cases of primary graft rejection, which led to implantation failure. These cases received hematopoietic and immune reconstitution following two haploidentical transplantations, with the mothers as the donors. One patient died of intracerebral hemorrhage on the 28th day after implantation due to 0% donor status, which resulted from implantation failure. Two patients died of infection and systemic failure on the 43th and 67th day, respectively, following implantation from a 0% donor.
Hematopoietic recovery information following implantation
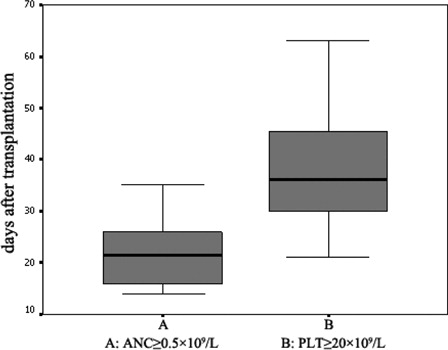
Following DUCBT transplantation, among the patients who achieved implantation, the median time to an ANC ≥0.5 × 109/L was 21 days (range: 14–35 days) following transplantation, and the median time to a platelet count ≥20 × 109/L was 36 days (range: 21–63 days) following transplantation ().
STR-PCR dynamic monitoring result following implantation
The STR-PCR analysis after implantation revealed the following results. In the early stage following transplantation, the grafts exhibited single or double donor–recipient chimerism. Following implantation, the vast majority of implantations were biased toward a single donor; one patient exhibited long-term proportional double chimerism with no recipient ingredient. On the 7th day after implantation, STR-PCR indicated five cases of single donor FDC and one case of double umbilical cord blood proportional mixed chimerism with no recipient ingredient. The remaining cases did not exhibit implantation. On the 14th day after implantation, we observed 24 cases of FDC, and the remaining five patients did not achieve implantation. On the 21st day after following implantation, there were 24 cases of FDC, and the remaining 5 patients did not achieve implantation. On the 30th day, the result was the same as for on the 21st day. On the 14th day following implantation in the 24 patients who achieved implantation, STR-PCR indicated single donor FDC in 23 cases and 1 case of double umbilical cord blood proportional mixed chimerism with no recipient ingredient. The remaining patients who did not achieve implantation, STR-PCR indicated that they did not achieve FDC on the 14th day after implantation. The consistency test results for graft chimerism and implantation status on the 7th, 14th, 21st, and 30th days after implantation are given in detail in Tables and .
Table 2. Chimeric status statistical analysis after implantation
Table 3. Consistency test result
DUCBT implantation kinetics research
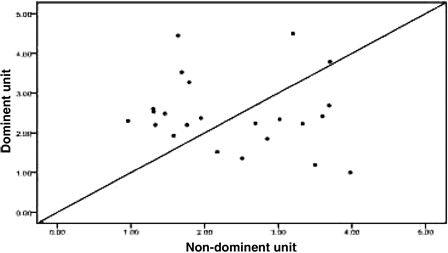
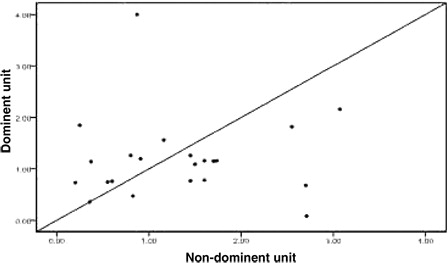
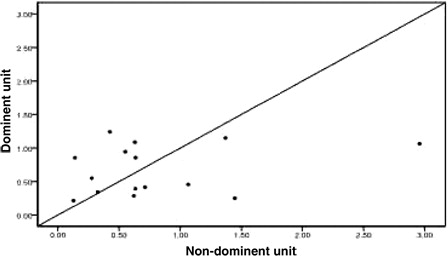
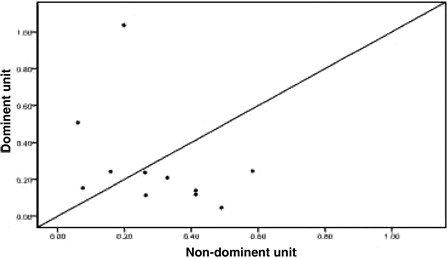
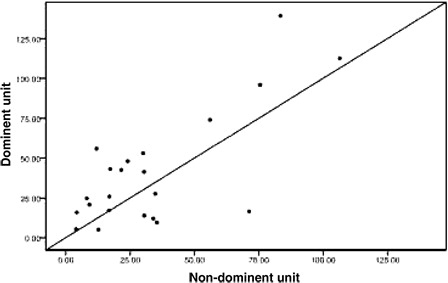
Twenty-four patients achieved implantation after transplantation, including one patient who exhibited proportional chimeric implantation with no recipient ingredient. For the 23 patients who achieved single-donor FDC we used the STR-PCR results to statistically compare the dominant unit and non-dominant blood units based on the following metrics: TNC count following thawing, CD34+ cell number following thawing, CFU and CFU-GM number prior to freezing, and CD3+ cell count following thawing (all values are given in terms of the recipient's weight in kilograms). We also analyzed the DUCBT implantation kinetics of the implanted units. All of these measures are collectively referred to as dominant unit characteristics. Owing to differences between the tests that are performed in the various cord blood banks in China and the failure of certain cord blood banks to determine CFU and CFU-GM counts prior to freezing, the data were incomplete. Twenty-three cases had data for TNC count following resuscitation; in 13 of these cases, the dominant unit count was greater than that for the non-dominant unit, whereas in 10 cases, the dominant unit count that was less than that for the non-dominant unit (). There were 22 cases for which CD34+ cell counts were available; in 9 of these cases, the dominant unit count was greater than that for the non-dominant unit, whereas in 12 cases, the dominant unit count was less than that for the non-dominant unit, and the two counts were the same for the remaining cases (). The CFU count prior to freezing was available for 15 cases; for eight of these cases, the dominant unit count was greater than that for the non-dominant unit, whereas in seven cases, the dominant unit count was less than that for the non-dominant unit (). The CFU-GM count prior to freezing was available for 11 cases; for four of these cases, the dominant unit count was greater than that for the non-dominant unit, whereas for seven cases, the dominant unit count was less than that for the non-dominant unit (). The CD3+ cell count following resuscitation was available for 22 cases; for 16 of these cases, the dominant unit count was greater than that of the non-dominant unit, whereas for 6 cases, the dominant unit count was less than that of the non-dominant unit (). We performed statistical analyses of these metrics separately to determine whether there were any differences between the dominant and the non-dominant units. For these analyses, the P values were 0.584, 0.322, 0.842, 0.534, and 0.082, respectively, and were not statistically significant.
Implantation follow-up information
GVHD occurrence
Of 29 patients, 12 patients suffered from acute GVHD (aGVHD), including 9 cases of grade I–II and 3 cases of grade III–IV. The rate of aGVHD incidence was 41.4%, whereas the incidence of chronic GVHD was 13.8% (four patients).
Cytomegalovirus infection following transplantation
Of 29 patients, 12 patients (41.4%) exhibited different degrees of cytomegalovirus infection.
Hemorrhagic cystitis occurrence
Of 29 patients, only one patient (3.4%) developed hemorrhagic cystitis.
Disease recurrence following transplantation
With respect to the disease diagnoses of the 29 patients prior to implantation, there were 9 high-risk patients, 5 patients with refractory diseases, and 5 patients with disease progression, accounting for 65.5% of the total number of patients. In total, five patients (17.2%) exhibited disease recurrence following the transplantation, including extramedullary relapse in three cases and bone marrow relapse in two cases.
Long-term survival
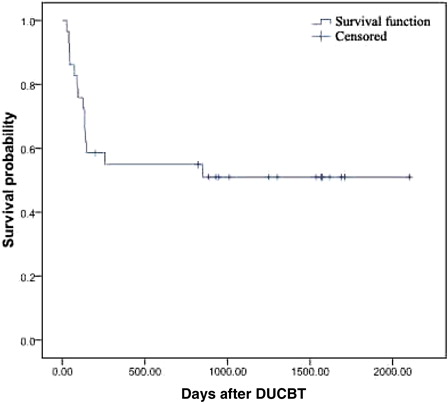
The follow-up of the 29 patients is ongoing. As of 1 December 2011, 15 patients had survived, for an overall survival rate of 51.0% (see survival curve in ). During the first 100 days following the transplantation, the related mortality rate was 24.1%. During the first 2 years following transplantation, the overall survival rate was 55%, and the disease-free survival rate was 36.9%. With respect to the deceased cases, seven died of pulmonary infection and respiratory failure, two died of aGVHD and systemic failure, one died of primary disease relapse, one died of FK506 poisoning, one died of a cerebral hemorrhage, one died of chronic severe hepatitis B, and one committed suicide.
Conclusion
| 1. | Following DUCBT, we used dynamic STR-PCR monitoring to demonstrate that DUCB chimerism is a dynamic process with respect to the chimeric contribution of the donor units. Following graft implantation, the vast majority of cases exhibited single-donor advantaged implantation; one case of long-term, two-donor with no recipient ingredient. was also observed. | ||||
| 2. | Based on the STR-PCR analysis, we determined that DUCB implantation was achieved 14 days following transplantation, and the STR-PCR results were consistent with successful implantation (kappa = 0.791, P = 0.001). Therefore, based on the STR-PCR results from the 14th day, we were able to perform early salvage treatment for those who failed to achieve 100% single-donor FDC, such as double haploidentical hematopoietic cell transplantation with the mother or the father as the donor. This procedure reduced related complications and mortality due to implantation failure, which are very important considerations in the context of clinical treatment. | ||||
| 3. | We performed comparisons and statistical analyses of the properties of the dominant and non-dominant units, including TNC count following resuscitation, CD34+ cell count following resuscitation, CFU and CFU-GM count prior to freezing, and CD3+ cell number following resuscitation; however, none of these metrics differed between the two types of units. DUCBT implantation kinetics therefore require further research. | ||||
Discussion
Unrelated umbilical cord blood hematopoietic stem cell transplantation began to be performed in the 1990s.
In addition to the rapid development of experimental technology, STR-PCR has become a highly sensitive and accurate method by which implantation can be ascertained following allogeneic hematopoietic stem cell transplantation. Upon the introduction of its use in clinical practice, researchers began to study DUCBT in comparison to single UCBT. Here, we used STR-PCR to monitor donor and recipient chimerism following transplantation in 29 patients who received DUCBT at a single center. The results were statistically analyzed and revealed that DUCBT resulted in implantation on the 14th day following transplantation; in most cases, single-donor advantaged implantation was observed. This result was crucial for the early determination of whether the DUCB implantation was successful and provided the basis for early clinical salvage treatment for patients. Thus, donor and recipient chimerism was clinically significant on the 14th day following transplantation; if, on the 14th day following DUCBT, the analysis of the chimerism status indicated that implantation had not occurred, the implantation was likely to fail. In this situation, one should consider other treatments, such as haploidentical hematopoietic stem cell transplantation with the mother or the father as the donor, which could lead to recovery of the patient's hematopoietic status and reduce transplant-related mortality. This was the case for two patients in the course of the study, for whom the STR-PCR analysis in the early stages post-transplantation indicated NDC. We therefore performed haploidentical hematopoietic stem cell transplantation with the mother as the donor, which helped the patients achieve timely hematopoietic and immune reconstitution, avoiding the death that would have resulted due to transplantation failure. We also observed that, among those patients who achieved one umbilical cord blood advantaged implantation on the 14th day following transplantation, one exhibited 0% chimerism on the 21st day based on STR-PCR analysis, which was followed by a failed secondary implantation. On the 33rd day, the patient received hematopoietic and immune reconstitution following haploidentical hematopoietic stem cell transplantation, with the mother as the donor. In another case, analysis on the 14th day following transplantation revealed donor–recipient chimerism. On the 21st day, 100% umbilical cord blood advantaged implantation was observed. However, long-term monitoring revealed that the donor ratio decreased gradually due to unknown causes, although this patient is currently alive. It is therefore crucial to monitor donor and recipient chimerism status following implantation. In cases of DUCBT, if DUCB long-term chimeric implantation is observed, it should be considered to represent successful implantation; however, this cannot be concluded on the 14th day following implantation. Given that long-term double chimerism appears only rarely, preoperative examination of the donor and stem cell collection should be performed to prepare for haploidentical hematopoietic stem cell transplantation with the mother or the father as the donor. Therefore, if DUCB chimeric implantation appears, it is better to prepare for the second-line haploidentical implantation treatment, for which STR-PCR monitoring can also be performed.
For 23 patients who achieved single-donor advantaged implantation, we compared and statistically analyzed the characteristics of the dominant and non-dominant units
These characteristics included the TNC count following resuscitation, CD34+ cell count following resuscitation, CFU and CFU-GM count prior to freezing, and CD3+ cell count following resuscitation. We determined that, for all of these donor unit characteristics, the value of dominant unit is higher than for the non-dominant unit; however, these differences were not statistically significant. Thus, DUCBT implantation kinetics remain uncharacterized and require further research. At present, it is believed that CD3+ cell counts are higher in the dominant cord blood unit. In this single-center study, we analyzed this metric for 22 cases; for 16 of these cases (72.7%), the CD3+ cell count of the dominant unit was greater than of the non-dominant unit, whereas for 6 cases (27.3%), the CD3+ cell count of the dominant unit was less than that of the non-dominant unit (P = 0.082). Therefore, we observed no statistically significant difference in this study, although this analysis would benefit from an increased sample size. Because various cord blood banks in China test for different cord blood characteristics, the CFU and CFU-GM counts prior to freezing were not consistently available for our analyses, which could have influenced the statistical results to some extent. However, we plan to examine additional clinical cases for further analysis of DUCBT implantation kinetics, and this continued study will be performed in cooperation with several research centers to provide the optimal choice for DUCB. The results of this collaboration will have broad clinical applications with respect to this procedure.
References
- Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Eng l J Med. 1989;321:1174–78.
- Tse W, Bunting KD, Laughlin MJ. New insights into cord blood stem cell transplantation. Curr Opin Hematol. 2008;15:279–84.
- Sanz MA, Sanz GF. Unrelated donor umbilical cord blood transplantation in adults. Leukemia. 2002;16:1984–91.
- Tse WW, Zang SL, Bunting KD, Laughlin MJ. Umbilical cord blood transplantation in adult myeloid leukemia. Bone Marrow Transplant. 2008;41:465–72.
- Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–85.
- Sanz GF, Saavedra S, Planelles D, Senent L, Cervera J, Barragan E, et al. Standardized, unrelated donor cord blood transplantation in adults with hematologic malignancies. Blood. 2001;98:2332–8.
- Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–75.
- Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–18.
- Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–47.
- Thiede C, Florek M, Bornhauser M, Ritter M, Mohr B, Brendel C, et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant. 1999;23:1055–60.
- Sufliarska S, Minarik G, Horakova J, Bodova I, Bojtarova E, Czako B. Establishing the method of chimerism monitoring after allogeneic stem cell transplantation using multiplex polymerase chain reaction amplification of short tandem repeat markers and Amelogenin. Neoplasma. 2007;54:424–30.
- Ariffin H, Daud SS, Mohamed Z, Ibrahim K, Lee TF, Chong LA. Evaluation of two short tandem multiplex systems for posthaematopoietic stem cell transplantation chimerism analysis. Singapore Med J. 2007;48:333.
- Kang HJ, Kho SH, Jang MK, Lee SH, Shin HY, Ahn HS. Early engraftment kinetics of two units cord blood transplantation. Bone Marrow Transplant. 2006;38:197–201.
- Claudio G. Brunstein, Juliet N. Barker, Daniel J. Weisdorf, Todd E. DeFor, Jeffrey S. Miller. Hematologic disease conditioning: impact on transplantation outcomes in 110 adults with Umbilical cord blood transplantation after nonmyeloablative. Blood. 2007;110:3064–70.
- Sharon Avery, Weiji Shi, Marissa Lubin, Anne Marie Gonzales, Glenn Heller, Hugo Castro-Malaspina. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117:3277–85.