Abstract
High-dose therapy with autologous stem cell transplant (ASCT) has been established as standard treatment for eligible patients with myeloma. However, whether this approach is still beneficial with new therapy is yet to be determined. Consolidation of effective therapy may be an alternative to ASCT following major response to initial induction. This retrospective case-series analysis included a total of 48 patients with newly diagnosed myeloma. All these patients achieved complete response or very good partial response to bortezomib-based induction and were eligible for ASCT; 24 of these patients proceeded with ASCT, and other 24 patients opted out of ASCT and received two additional cycles of bortezomib therapy as consolidation. With a median follow-up of 28.5 months in ASCT group and 29 months in non-ASCT group, no significant difference was seen in progression-free survival, 39 versus 32 months, P = 0.82. Median overall survival had not been reached, and the estimated 3-year overall survival rates were 87.5 and 67.5% in ASCT and non-ASCT, respectively, P = 0.97. This study provides an initial assessment of survival outcome of ASCT in comparison with non-ASCT consolidation. The additional study is required to establish the efficacy of non-ASCT consolidation.
Introduction
The treatment of multiple myeloma (MM) has been evolving rapidly. The response and survival in MM patients have improved significantly in recent years.Citation1–Citation3 It has been demonstrated that MM patients diagnosed in the last decade had a median overall survival (OS) of nearly 4 years, a 50% improvement compared with the previous two decades.Citation1 This progress is largely due to the introduction of high-dose therapy plus autologous stem cell transplantation (ASCT) and new therapeutic agents including the immunomodulatory drug thalidomide and lenalidomide, and the proteosome inhibitor bortezomib. These novel agents are highly active and have been increasingly used as front-line therapy.Citation1–Citation5
Before the advent of novel therapies, the high rates of complete response (CR) and other major responses were achieved mostly by high-dose melphalan plus ASCT.Citation6,Citation7 This strategy improves the response by increasing dose intensity with ASCT support. In the ASCT setting, achievement of CR has been shown to be a key factor for prolonged survival and long-term disease control.Citation8,Citation9 The ASCT approach is currently considered the standard care for eligible patients.Citation10,Citation11 However, since new induction regimens are offering similar response rates, and novel therapies early in the course of MM may have an impact on the course of the disease, the role of ASCT in MM treatment needs to be re-evaluated.Citation12,Citation13 A particular issue is whether ASCT is still beneficial to the patients who have achieved ≥VGPR (very good partial response) with new induction regimens. We have used bortezomib-based regimens as front-line therapy for newly diagnosed MM patients in our center since 2006. For the patients of ≤65 years of age who achieve ≥VGPR, ASCT or additional induction was administered as consolidation therapy. Here, we report the results of a case-series analysis comparing the outcome of ASCT versus non-ASCT following the induction therapy.
Patients and methods
Patients and data collection
This is a retrospective case-series analysis of the patients who were treated initially with bortezomib-based induction. The patients in this study were selected from those newly diagnosed MM and treated in our center between January 2006 and October 2011. During this period, a total of 125 patients were treated with bortezomib-based inductions in our center. Among these patients, 73 (58.4%) achieved CR or VGPR with the induction therapy. Out of the 73 patients who achieved ≥VGPR, 48 were ≤65 years of age and considered eligible for ASCT.
According to our local practice guidelines, ASCT was offered to all eligible patients, and non-transplant consolidation with additional induction regimens were administered to the patients who opted out of ASCT. In this study, 24 patients underwent ASCT, and other 24 patients declined ASCT for the concerns of high-dose therapy toxicity, quality of life, and the cost of ASCT. There was no bias by physicians in selection for ASCT. Survival outcomes of the two groups of ASCT and non-ASCT were evaluated with data from medical record review and follow-up results.
Treatment
All of the patients in this analysis received induction therapy with one of the three bortezomib-based induction regimens: bortezomib–dexamethasone (VD) consisting of 3-week cycles of bortezomib 1.3 mg/m2, administered intravenously on days 1, 4, 8, and 11 plus oral dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, 12; bortezomib–dexamethasone–doxorubinxin (PAD) consisting of intravenous bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 plus oral dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, 12 and doxorubicin 9 mg/m2 intravenously on days 1–4; and bortezomib–thalidomide–dexamethasone (VtD) consisting of intravenous bortezomib 1.3 mg/m2, on days 1, 4, 8, and 11 plus oral dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, 12 and thalidomide 100 mg daily through the 21-day cycle.
A total of 24 patients proceeded with ASCT. Conditioning for ASCT was 200 mg melphalan administered intravenously on day −2 (n = 11), or melphalan plus bortezomib 1 mg/m2 intravenously on days −6, −3, +1, and +4 (n = 13). Stem cells were mobilized with cyclophosphamide 4 g/m2 plus G-CSF 5 µg/kg/day, and peripheral blood stem cells (≥2 × 106 CD34+ cells/kg) were infused on day 0.
All the patients received thalidomide 100 mg daily as maintenance therapy. In the ASCT group, thalidomide started 2 months after complete hematopoietic engraftment. In the non-ASCT group, thalidomide was given after consolidation with additional two cycles of bortezomib regimens. Thalidomide maintenance continued during the follow-up period. All of the patients on thalidomide received aspirin daily for deep vein thrombosis prophylaxis. No assessment was performed for improvement in response to thalidomide.
Assessments
Treatment response and disease progression were determined in accordance with the International Myeloma Working Group uniform response criteria for disease progression and relapse. CR was defined as no detectable M-protein by electrophoresis and immunofixation with <5% plasma cells in the bone marrow; VGPR was defined as at least 90% reduction in serum M-protein plus urine M-protein level <100 mg per 24 hours. Stringent CR was not used in this study as patients were not assessed for free light chain. Responses were assessed after each induction cycle and every 3 months after ASCT. All toxicities were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. The progression-free survival (PFS) was calculated from the start of induction therapy to the time of progression, relapse, death from any cause, or the date the patient was last known to be in remission. OS was defined as the period from the start of treatment to the date of the last follow-up or death from any cause.
Statistical analysis
The estimates of PFS and OS were calculated using the Kaplan–Meier method. Comparisons of PTF and OS were performed using log-rank test. Differences in response were compared using the XCitation2 test or Fisher's exact test. Differences in the means of continuous measurements were tested by Student's t-test and checked using the Mann–Whitney U-test. Comparisons were two-sided unless otherwise specified, using P values less than 0.05 to determine significance. All statistical analyses and survival curves were prepared by using SPSS 11.5 software.
Results
Patient characteristics and response to induction therapy
All of the patients in this study were newly diagnosed and symptomatic MM. summarizes the patient characteristics in the ASCT and non-ASCT groups. All these patients were offered ASCT after achieving CR or VGPR. ASCT or non-ASCT was based on patient's decision. There was no case selection bias for patients. Overall, the demographics and base line disease characteristics were comparable between the ASCT and non-ASCT patients, with no statistical difference in the parameters between the two groups. Cytogenetic data were not included in the analysis because only nine patients were assessed using interphase FISH tests. Although positive findings for the del(17p) and the t(14;14) were seen in both groups, the results were limited and not used to determine the distribution of cytogenetic aberrations between two groups. There were four patients who presented with renal failure, two in the ASCT group and two in the non-ASCT group.
Table 1. Patient base-line characteristics
All the patients were initially treated with the bortezomib-based regimens as front-line induction therapy. In the ASCT group, 11 patients achieved CR and 13 patients had VGPR after a median of four cycles of induction therapy with the bortezomib-based regimens (VtD n = 9, PAD n = 11, VD n = 4). In the non-ASCT group, nine patients achieved CR and 15 achieved VGPR after a median of four cycles of induction therapy (VtD n = 12, PAD n = 9, VD n = 3). Notably, serum creatinine levels returned to normal in all four patients with renal failure.
Consolidation with ASCT or bortezomib-based therapy
Following CR or VGPR, 24 patients proceeded with high-dose melphalan and ASCT, as described above. Complete hematopoietic engraftment was achieved in all of these patients. The median time to neutrophil and platelet regeneration was 12 days (range: 9–18 days) and 13 days (range: 9–21 days), respectively. An upgraded response to ASCT was seen in nine patients who had VGPR with initial inductions, including four patients with VtD, three patients with PAD, and two patients with VD, and they achieved CR status following ASCT. There were no evident differences between those conditioned with melphalan alone and those with melphalan plus bortezomib.
For the patients who declined ASCT, additional two cycles of the induction regimen were administered as consolidation. Thus, including the consolidation, the non-ASCT group patients received a median of six cycles of therapy (range: 5–10). Further response was seen with an improvement to CR in four of the 15 patients. summarizes the overall response to induction and results after ASCT or non-ASCT consolidation.
Table 2. Responses to induction, ASCT, and consolidation
Treatment-related toxicities
Induction therapy was well tolerated by most patients included in this study. The common treatment-related adverse events encountered during induction therapy are summarized in . The most common toxicities were peripheral neuropathies and infections. Bortezomib was held for 1 week during induction therapy for Grade 3 peripheral neuropathies in five patients (three in ASCT and two in non-ASCT). But there were no withdrawals or dose reductions of bortezomib. Among other toxicities, three (6.25%) developed DVT, and eight (16.7%) developed herpes zoster. There was no statistically significant difference in each of the adverse events between the two groups.
Table 3. Adverse events during induction therapy
In the ASCT group, 13 patients received melphalan plus bortezomib as conditioning regimen. It is noteworthy that peripheral neuropathy was present in nine patients before ASCT, and with melphalan/bortezomib treatment, aggravation of peripheral neuropathy occurred only in one of these patients.
Outcome analysis
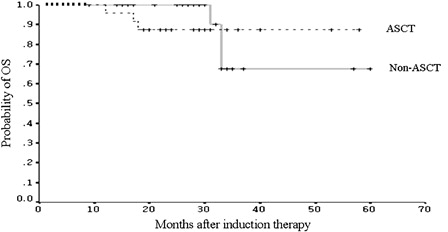
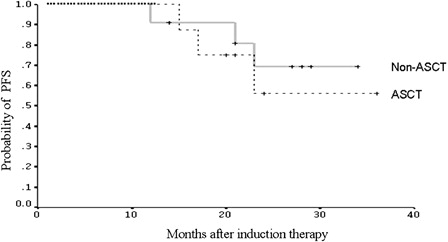
The time-to-event data were collected with a median follow-up of 29 months (range: 9–60 months) in the non-ASCT group and 28.5 months (range: 12–58 months) in the ASCT group, respectively. shows the comparison of PFS between the two groups. There was no significant difference in median PFS (32 in non-ASCT group versus 39 months in ASCT group; P = 0.82). In the non-ASCT group, 12 patients had relapsed. Although these patients could receive HDT/ASCT at relapse, they all chose salvage chemotherapy for the same reasons that they declined upfront ASCT. The median OS from the initiation of induction therapy had not been reached in either group with the follow-up duration. The estimated 2- and 3-year OS rates in the non-ASCT group were 90.0 (SE 9.49%) and 67.5% (SE 15.51%), respectively. In the ASCT group, the estimates of 2- and 3-year OS were the same at 87.5% (SE 6.75%). There was no statistical difference in OS estimates between the two groups (P = 0.97) (). Because of the small numbers of patients, no further analysis was performed to compare the response rates and outcomes in the different induction protocols.
Figure 1. Progression-free survival (PFS) of the patients who underwent ASCT (n = 24) and the patients who received non-ASCT consolidation (n = 24) following CR or VGPR.
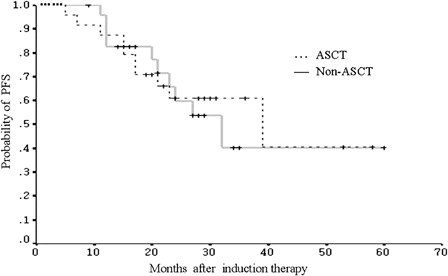
A total of 19 patients, including 11 in the non-ASCT group and eight in the ASCT group, were identified as having ISS stage III disease before induction therapy. Within this subgroup, the estimated 2-year PFS was 69.3% in patients without ASCT, compared with 56.3% in patients with ASCT. The difference between the two groups was not statistically significant (P = 0.62) ().
Discussion
The objective of this analysis was to evaluate ASCT versus non-ASCT consolidation following a major response to bortezomib-based induction. This study included only the patients who had a major response to initial induction, and CR and VGPR were grouped together in the outcome analysis. The survival data showed comparable PFS and OS between the patients with ASCT and those without ASCT. Our strategy for non-ASCT patients was to consolidate with additional two cycles of the induction therapy. Further improvement was seen with either ASCT or non-ASCT consolidation, with nine of 13 patients in ASCT and four of 15 patients in non-ASCT upgraded from VGPR to CR. However, it remains to be determined whether patients achieving CR following ASCT or non-ASCT consolidation would have better outcomes in comparison to those with VGPR. The analysis was limited by the small numbers of VGPR patients remaining in both groups. This issue needs to be addressed with sufficient number of patients in future studies.
Currently, treatment decision in newly diagnosed MM is based on whether the patient is eligible for transplantation. For eligible patients, which include those under the age of 65–70 years and without comorbidities,Citation14,Citation15 ASCT after a three-to-four-cycle induction is considered the standard of care. This approach is based largely on the results of the Intergroupe Francophone du Myeloma and other randomized trials that demonstrated a survival benefit of ASCT compared to conventional therapy.Citation6,Citation7 However, when these trials were performed, few patients achieved VGPR or CR before ASCT. It was also shown by the PETHEMA trial that ASCT did not have a significant impact on PFS or OS for patients who have responded to initial chemotherapy.Citation16 Therefore, the rationale for ASCT may be lost with the use of highly effective new therapies. Given the comparable outcome of this study, and along with the costly resources and toxicities associated with high-dose therapy, ASCT may be reserved mainly for patients refractory to induction therapy or delayed until relapse.
The bortezomib-based induction regimens used in this study have been previously reported as effective front-line therapies. Harousseau et al.Citation17 demonstrated that VD, as pretransplant induction therapy, was superior to vincristine, adriamycin, and dexamethasone. PAD was shown to be a highly effective induction regimen for MM patients who were eligible for ASCT. This regimen produced an overall response rate of 95%, including 24% CR and 62% combined CR/VGPR.Citation18,Citation19 The triple combination VtD was initially used in the setting of recurrent disease, and has subsequently shown good efficacy in front-line patients eligible for ASCT. In a group of 34 patients, this regimen gave an overall response rate of 94%, with 56% patients achieving ≥VGPR. Interestingly, there were no significant differences in PFS (27.4 versus 23.5 months) or 2-year survival (80 versus 90%) between patients with ASCT and those without ASCT.Citation20 More recently, the Intergroupe Francophone du Myeloma trial showed that VtD regimen remains active with a 49% CR/VGPR rate.Citation21 Most recently, Cavo et al.Citation22 demonstrated in a randomized study the efficacy of VtD when re-administrated as consolidation therapy after ASCT. VtD consolidation significantly increased CR/nCR rates and contributed to improve clinical outcomes.
In this analysis, the bortezomib-based regimens demonstrated similar response rates to the results from published series. Among the total 125 patients in the front-line setting, the combined CR and VGPR rate was 58%. As the objective of this study was to evaluate the effect of ASCT with bortezomib consolidation, the inclusion of patients was based on the response and eligibility for ASCT. We recognize that this study is limited by the small number of the patients and lack of molecular cytogenetic risk-stratification. There is a possibility of imbalance in cytogenetic variables between two groups. Nonetheless, this study only included the patients who had achieved a major response with the standard induction regimens. There was no selection bias for patients to undergo ASCT or non-ASCT consolidation. The base line disease characteristics were found to be comparable between the groups. There were 19 patients with ISS stage III disease. There appeared no significant impact of ASCT on PFS when these patients were analysed as a subgroup (). However, owing to the small number of cases in this analysis, the result should be interpreted with caution.
In conclusion, it is relevant to re-evaluate the role of ASCT in MM patients with the advent of novel therapies. This study provides data that shows no significant survival benefit of ASCT in comparison with non-ASCT consolidation. The finding suggests that consolidation with effective induction regimens may yield similar outcome to ASCT following major response to induction.
Acknowledgements
This study was performed as a joint project. Wenming Chen and Chen Wang were principal investigators at the Beijing Chaoyang Hospital and Mount Sinai Hospital Toronto, respectively.
References
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
- Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9:135–43.
- Moreau P, Avet-Loiseau H, Harousseau JL, Attal M. Current trends in autologous stem-cell transplantation for myeloma in the era of novel therapies. J Clin Oncol. 2011;29:1898–906.
- Laubach JP, Schlossman RL, Mitsiades CS, Anderson KC, Richardson PG. Thalidomide, lenalidomide and bortezomib in the management of newly diagnosed multiple myeloma. Expert Rev Hematol. 2011;4:51–60.
- Ludwig H, Beksac M, Bladé J, Boccadoro M, Cavenagh J, Cavo M, et al. Current multiple myeloma treatment strategies with novel agents: an European perspective. Oncologist. 2010;15:6–25.
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma: Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–7.
- Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. Medical Research Council Adult Leukaemia Working Party: high dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83.
- Van de Velde HJK, Liu X, Chen G, Cakana A, Draedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92:1399–406.
- Lahuerta JJ, Mateos MV, Martinez-Lopez J, Rosiñol L, Sureda A, de la Rubia J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–82.
- Harousseau JL, Moreau P. Autologous hematopoietic stem-cell transplantation for multiple myeloma. N Engl J Med. 2009;360:2645–54.
- Palumbo A, Rajkumar SV. Treatment of newly diagnosed myeloma. Leukemia. 2009;23:449–56.
- Bensinger W. Stem-cell transplantatin for multiple myeloma in the era of novel drugs. J Clin Oncol. 2008;26:480–92.
- Kumar S. Multiple myeloma – current issues and controversies. Cancer Treat Rev. 2010;36( Suppl 2):S3–11.
- Cavo M, Rajkumar SV, Palumbo A, Moreau P, Orlowski R, Bladé J, et al. International Myeloma Working Group: International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117:6063–73.
- Engelhardt M, Kleber M, Udi J, Wäsch R, Spencer A, Patriarca F, et al. Consensus statement from European experts on the diagnosis, management, and treatment of multiple myeloma: from standard therapy to novel approaches. Leuk Lymphoma. 2010;51:1424–43.
- Bladé J, Rosiñol L, Sureda A, Ribera JM, Díaz-Mediavilla J, García-Laraña J, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–9.
- Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621–9.
- Popat R, Oakervee HE, Hallam S, Curry N, Odeh L, Foot N, et al. Bortezomib, doxorubicin and dexamethasone (PAD) front-line treatment of multiple myeloma: updated results after long-term follow-up. Br J Haematol. 2008;141:512–6.
- Oakervee HE, Popat R, Curry N, Smith P, Morris C, Drake M, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol. 2005;129:755–62.
- Kaufman JL, Nooka A, Vrana M, Gleason C, Heffner LT, Lonial S. Bortezomib, thalidomide, and dexamethasone as induction therapy for patients with symptomatic multiple myeloma: a retrospective study. Cancer. 2010;116:3143–51.
- Moreau P, Avet-Loiseau H, Facon T, Attal M, Tiab M, Hulin C, et al. Brtezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752–8.
- Cavo M, Pantani L, Petrucci MT, Patriarca F, Zamagni E, Donnarumma D, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy following autologous hematopoietic stem-cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120:9–12.