Abstract
Background
Although Epstein-Barr virus (EBV) and cytomegalovirus (CMV) are known to trigger immune thrombocytopenia (ITP), the manifestations of EBV and CMV in ITP spleen tissues continue to be poorly understood.
Methods
Our research retrospectively reviewed a total of 42 ITP patients and 20 healthy control cases from the West China Hospital of Sichuan University from 2008 to 2012. Patients' characteristics, preoperative platelet counts, and the expression of EBV and CMV in spleen tissue were analyzed.
Results
No statistically significant differences were identified for age and gender between the ITP group and the healthy control group. The comparison of these two categories (the ITP group and the control group) showed substantial variations in the expression of EBV and CMV: nine (21.4%) and eight (19%) ITP patients had a positive expression of EBV and CMV, compared with none and one patient in the control group, respectively. In the EBV-ITP group, patients with a positive EBV expression revealed appreciably decreased preoperative platelet count compared with patients with a negative EBV expression. No other statistically significant difference was found in the CMV group.
Conclusion
We have demonstrated the presence of both EBV and CMV infections in the spleen tissue of ITP patients. EBV infections were implicated in the reduced platelet counts in ITP.
Introduction
Immune thrombocytopenia (ITP) is known to be an autoimmune disorder that is identified by its early exploitation of platelets that are triggered by the reticuloendothelial system following sensitization by antiplatelet glycoprotein autoantibodies.Citation1 Other components of the disorder may include complement-mediated lysis, inadequate thrombopoiesis, or a viral infection that has a detrimental effect on the body's defense mechanisms.Citation2 Even so, the pathogenesis continues to be poorly understood.
The autoimmune system's course of action is assumed to occur within the spleen.Citation3 Scientific studies have revealed that viruses, for instance cytomegalovirus (CMV) and the Epstein-Barr virus (EBV), may result in patients with ITP.Citation4 In viral disease, the mechanism of thrombocytopenia appears to be multifactorial. The mechanism is usually related to possible deterioration of the immune system that may be caused by antiplatelet antibodies or immune complexes, faulty platelet generation, or an altered reticuloendothelial performance, which are the most common explanations for thrombocytopenia-associated viral diseases.Citation5,Citation6 Nevertheless, no analysis has revealed EBV protein expression and CMV late protein expression in the spleen.
We used immunohistochemistry (IHC) to analyze the occurrence of virus-associated ITP in patients from Sichuan University. Although a couple of scientific studies of EBV and CMV expression in ITP were found in the literature, no study has previously assessed their expression in splenic tissue. This study comprises of a small group of patients for whom the clinical epidemiology, research laboratory attributes, and the impact of CMV- and EBV-associated ITP is frequently considered. This study attempts to demonstrate that a substantial percentage of the ITP cases in China are associated with EBV and CMV.
Methods
This study was a retrospective report on 42 patients who were clinically diagnosed with ITP and received a laparoscopic splenectomy at Sichuan University from 2008 to 2012 (the ITP group). The demographics and clinical features of these patients are summarized in . The 42 ITP cases were diagnosed in the Department of hematology in accordance with the guidelines of the American Society of Hematology and the United Kingdom's practice for management of ITP. All the patients were previously treated with corticosteroids (prednisone: 1 mg/kg/day) for 4–6 months before surgery. A laparoscopic splenectomy was indicated in all cases because their platelet counts (PLTs) increased to unsatisfactory levels. At the same time, we identified an additional 20 overall healthy control patients who had undergone a splenectomy for trauma (the control group). The thrombocytopenia levels have been divided into four grades, including mild (a PLT more than 50 × 109/l), moderate (a PLT between 25 × 109/l and 50 × 109/l), severe (a PLT between 10 × 109/l and 25 × 109/l), and very severe (a PLT lower than 10 × 109/l). Informed consent was received from the parents of patients who were younger than 12 years of age and from all patients who were older than 12 years of age. This study was approved by the Ethics Committee of Sichuan University.
Table 1. Patients' characteristics
Prior to a laparoscopic splenectomy, each of the affected individuals was administered an increased dose of steroids and 10 g of intravenous immunoglobulin on a daily basis for 3 days.
Immunohistochemical staining for EBV and CMV
Immunohistochemical staining was performed according to the manufacturer's instructions. Briefly, 4 µm tissue sections from each representative area of the specimen were placed onto glass slides. After removing the paraffin using xylene twice at 15 minutes, the slides were rehydrated through a graded ethanol series (100–80%) and washed with distilled water. For antigen retrieval, tissue sections were boiled in 10 mM citrate buffer with a pH of 6.0 for 20 minutes, followed by cooling at room temperature for 20 minutes. After being washed in phosphate-buffered saline (PBS) three times, the endogenous peroxidase activity was suppressed using 3% hydrogen peroxide for 15 minutes. Next, the sections were incubated at 37°C for 30 minutes with normal goat serum and then incubated overnight at 4°C with a primary antibody (mouse anti-EBV cat#: ZM-0105; mouse anti-CMV (late antigen) cat#: ZM-0078; Zhongshan Goldenbridge Biotechnology, Beijing, China). The negative control was concomitantly processed by omitting the primary antibody, using PBS instead. Following incubation overnight, the sections were washed three times in PBS. Then, secondary antibodies (cat#: SP-9000; Zhongshan Goldenbridge Biotechnology) were applied to the sections at 37°C for 40 minutes. After washing three times, the sections were treated with horseradish peroxidase streptavidin complex (cat#: SP-9000; Zhongshan Goldenbridge Biotechnology) at 37°C for 30 minutes, diluted as recommended by the manufacturer. Finally, the sections were visualized using a DAB coloration reagent. The sections were rinsed in water, counterstained with hematoxylin, dehydrated, and mounted in a permanent mounting medium.
Measurements of FCGR expression by IHC
The IHC slides were examined independently by two investigators (X.X. Wei and J. Zhou) who were blinded to the clinicopathological and biological information. Each investigator estimated the intensity of staining in the entire section. The two investigators reevaluated the slides together if a discrepancy in the individual scores appeared to reach a consensus before combining the individual scores.
Statistical analysis
The data were analyzed using the standard version of SPSS for Windows version 16.0 (SPSS, Chicago, IL, USA). Differences between variables were compared using the parametric Student's t-test and the chi-square test. A P value <0.05 was considered statistically significant for all tests.
Results
Clinical symptoms
The ITP group had significantly more females than males and a much lower preoperative PLT compared with the control group (). No statistically significant difference was found for age. All the patients received pulse treatment before surgery. Seven days after surgery, no difference was found in the PLTs between the two groups.
Epstein-Barr virus in ITP
Nine patients from the ITP group had a positive EBV expression, whereas no patients in the control group had a positive EBV expression ( and ). In the ITP group, one patient had severe thrombocytopenia and the others experienced very severe thrombocytopenia. Despite the fact that no statistically significant variation was found in the PLT level of the ITP patients in the EBV (+) subgroup and the EBV negative (−) subgroup, the EBV (+) subgroup exhibited a preoperative PLT that was considerably reduced compared with the EBV (−) subgroup (3.78 ± 3.4 × 109/l vs 12.3 ± 12.1 × 109/l, P = 0.045). No large differences were found in the age and sexual categories. At postoperative 3 and 7 days, no variations in the PLT were observed that indicated statistically significant differences between the two subgroups (P = 0.551 and P = 0.920, respectively).
Figure 1. Negative: non-staining in macrophages. Positive: staining as yellow and/or brown in macrophages.
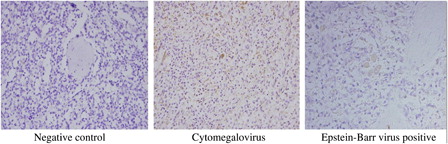
Table 2. Comparison between the EBV (+) subgroup and the EBV (−) subgroups
Cytomegalovirus in ITP
Eight patients in the ITP group had a positive CMV expression, and one patient in the control group had a positive CMV expression ( and ).
Table 3. Comparison between the CMV (+) subgroup and the CMV (−) subgroup
In the ITP group, two patients had moderate thrombocytopenia, one had severe thrombocytopenia, and five had very severe thrombocytopenia. No statistically significant variation was observed in the distribution of severe ITP patients between the CMV (+) subgroup and the CMV (−) subgroup, age, and gender. According to preoperative, postoperative 3 and 7 days, no statistically significant differences in the PLT were found regarding the two subgroups (P = 0.882, P = 0.527, and P = 0.508, respectively).
Discussion
ITP refers to diminished platelet numbers secondary to platelet devastation and diminished platelet manufacturing.Citation7 Some scientific research suggests that a pathogen causes an infection, including EBV, CMV, hepatitis virus, and HIV, as an explanation for ITP.Citation8,Citation9 Various components that play a role in platelet damage in ITP have previously been published.Citation10 The appearance of the autoantibodies after viral infection has previously been used in an attempt to clarify either specifically or generally a number of mechanisms.Citation11 These infections cause an autoimmune response in opposition to the platelets, whereas microbe infections can be temporary and apparently are frequently atypical or naturally severe. A virus–antivirus complex could continue with the surface of platelets, destroying them and eliminating them through the reticuloendothelial system. In addition, a virus particle may be a specific consequence of the bone marrow's megakaryocytes that encourage the formation of malfunctioning platelets.Citation12 Platelets that are slightly malfunctioning may perhaps energize the enhancement of the autoantibodies. The virus may depress T-cell function, with an accompanying depression of cell-mediated defenses.
In a subset of affected patients, the precise pathogen can be identified; for instance, EBV implied an etiological factor for causing virus-like infections in ITP patients.Citation13 Research on EBV infections of the spleen is, even so, exceptional. With the current research, specific medical symptoms and research laboratory data of the EBV-negative or EBV-positive affiliated groups yield similar results with the exception of the preoperative PLTs. The percentage of ITP patients with the optimal expression of EBV was 21.4%.
China is definitely an endemic area for CMV. The majority of people in China contracted these infections at an early age but do not exhibit symptoms. The likelihood of human CMV infections in China reaches a frightening number. The positive rate of CMV immunoglobulin G is greater than 80% in Chinese children.Citation14 This virus can survive in the body for an extended time and trigger significant complications. ITP is among the most commonly encountered hemorrhagic health conditions. Recently, the combined knowledge of immunology and molecular biology have suggested that CMV infection equally both triggers and perpetuates ITP in conjunction with a number of situations, for example, as in an immediate, cellular injury.Citation15,Citation16 Many experts have reported that human CMV can infect the megakaryocytes as well as their precursors in vitro by inhibiting their proliferation, which may stimulate the adulthood retention of megakaryocytesCitation17 or simply depress the bone marrow.Citation18 The second mechanism, theoretically, is the immunological abnormalities resulting in the diminishment of blood platelets. Abundant research indicates that gB can encourage the production of autoantibodies.Citation19,Citation20 Human CMV DNA reproduction continues to be extensively recorded in ITP patients; however, no reports focus on the expression of human CMV late-antigen protein in the spleen.
The prevalence of positive CMV antibodies was 87.5% in the adults available for this study.Citation21 In this study, the IHC-positivity rate for CMV was 19% in the ITP patients. This is comparable with another study on Chinese ITP-affected individualsCitation14 reporting an IHC-positivity rate of 19.11%. This implies that CMV may be a significant cause of ITP, and CMV markers should be investigated for all ITP-affected individuals.
In conclusion, 21.4 and 19% of the ITP patients in this study are linked to the EBV and CMV expression in the spleen's tissue, respectively. EBV-positive patients had a significantly decreased preoperative PLT compared with EBV-negative patients.
Acknowledgements
This study was approved by the West China Hospital, Sichuan University.
Zhong Wu and Jin Zhou contributed equally to this work.
References
- Cines DB, Blanchette S. Immune thrombocytopenic puerperal. New Engl J Med. 2002;346:995–1008.
- Taub JW, Warrier I, Holtkamp C, Beardsley DS, Lusher JM. Characterization of autoantibodies against the platelet glycoprotein antigens IIb/IIIa in childhood idiopathic thrombocytopenic purpura. Am J Haematol. 1995;48:104–7.
- Kuwana M, Okazaki Y, Ikeda Y. Splenic macrophages maintain the anti-platelet autoimmune response via uptake of opsonized platelets in patients with immune thrombocytopenic purpura. J Thromb Haemost. 2009;7:322–9.
- Wright JF, Blanchette VS, Wang H, Arya N, Petric M, Semple JW. Characterization of platelet-reactive antibodies in children with varicella-associated acute immune thrombocytopenic purpura (ITP). Br J Haematol. 1996;95:145–52.
- Leissinger CA. Platelet kinetics in immune thrombocytopenic purpura and human immunodeciency virus thrombocytopenia. Curr Opin Hematol. 2001;8:299–305.
- Weinblatt ME. Immune thrombocytopenic purpura evolving into aplastic anemia in association with Epstein Barr virus infection. Am J Pediatr Hematol Oncol. 1991;13:465–9.
- DiMaggio D, Anderson A, Bussel JB. Cytomegalovirus can make immune thrombocytopenic purpura refractory. Br J Haematol. 2009;46:104–12.
- Sipahi T. Human parvovirus B19 associated with idiopathic thrombocytopenic purpura. Pediatr Hematol Oncol. 2005;22:345–6.
- Rajan SK, Espina BM, Liebman HA. Hepatitis C virus-related thrombocytopenia: clinical and laboratory characteristics compared with chronic immune thrombocytopenic purpura. Br J Haematol. 2005;129:818–24.
- Rodrı´guez-Pinilla SM, Barrionuevo C, Garcia J, Martı´nez MT, Pajares R, Montes-Moreno S, et al. EBV-associated cutaneous NK/T-cell lymphoma: review of a series of 14 cases from Peru in children and young adults. Am J Surg Pathol. 2010;34:1773–82.
- Yenicesu I, Yetgin S, Ozyurek E, Aslan D. Virus-associated immune thrombocytopenic purpura in childhood. Pediatr Hematol Oncol. 2002;19:433–7.
- Cine DB, Bussel JB, Liebman HA, Luning Park ET. The ITP syndrome: pathogenic and clinical diversity. Blood 2009;113:6511–21.
- Jin CQ, Liu F, Dong HX, Zhang J, Zhou JW, Song L, et al. Type 2 polarized immune response holds a major position in Epstein-Barr virus-related idiopathic thrombocytopenic purpura (EBV-ITP). Intern J Lab Hematol. 2011;34:164–71.
- Zhong SY, Lan FT, Chao CZ, Ji YZ, Zheng YZ. Cytomegalovirus-associated idiopathic thrombocytopenic purpura in Chinese children. Scand J Infect Dis. 2008;40:922–7.
- Wei SH, Ho MC, Ni YH, Lin DT, Lee PH. Cytomegalovirus-associated immune thrombocytopenic purpura after liver transplantation. J Formos Med Assoc. 2007;106:327–9.
- Alliot C, Barrios M. Cytomegalovirus-induced thrombocytopenia in an immunocompetent adult effectively treated with intravenous immunoglobulin: a case report and review. Hematology 2005;10:277–9.
- Levy AS, Bussel J. Immune thrombocytopenic purpura: investigation of the role of cytomegalovirus infection. Br J Haematol. 2004;126:622–3.
- Crapnell K, Zanjani ED, Chaudhuri A, Ascensao JL, St Jeor S, Maciejewski JP. In vitro infection of megakaryocytes and their precursors by human cytomegalovirus. Blood 2000;95:487–93.
- Lipes J, Skamene E, Newkirk MM. The genotype of mice influences the autoimmune response to spliceosome proteins induced by cytomegalovirus gB immunization. Clin Exp Immunol. 2002;129:19–26.
- Newkirk MN, van Venrooij WJ, Marshall GS. Autoimmune response to U1 small nuclear ribonucleoprotein (U1 snRNP) associated with cytomegalovirus infection. Arthritis Res. 2001;3:253–8.
- Ustacelebi S, Koksal I, Canturk H, Saify SJ, Ersoz D, Sellioglu B. Detection of antibodies against TORCH agents during pregnancy. Mikrobiyol Bul. 1986;20:1–8.