Abstract
Retinol-binding protein (RBP) has been used as a nutritional index for children with acute myeloid leukaemia (AML) in previous studies. However, no studies have yet examined RBP levels in AML patients from all age groups. In this study, AML patients presented with lower RBP concentrations than healthy control subjects and patients with benign haematopathies. A negative association was observed between serum RBP level and peripheral white blood cell count in M4 and M5 AML patients. Moreover, patients carrying the FLT3-ITD mutation and young patients had lower RBP levels than those lacking this mutation and elderly patients. In conclusion, these observations suggest that aberrant retinol levels may be associated with AML.
Introduction
Retinol-binding protein (RBP) is a single polypeptide chain protein with a molecular mass of 21 kDa, which is classified under the prototypical lipocalin family due to its similarity with other lipid-binding proteins.Citation1–Citation2 RBP is mainly synthesized in the liver and is secreted bound to retinol along with transthyretin (also called prealbumin, PA) (molar ratio, 1:1:1).Citation1,Citation3,Citation4 Binding of RBP to PA serves to prevent the loss of the smaller protein from the circulation by filtration in the renal glomeruli.Citation1,Citation4 Therefore, the ternary PA–RBP–retinol complex serves to transport retinol through circulation, and deliver it from its storage site in the liver to target tissues.Citation1,Citation3,Citation4 Thus, RBP maintains the level of free retinol in the plasma,Citation1 and the membrane RBP receptor (STRA6) transfers vitamin A (retinol) from RBP, its blood carrier, into cells.Citation3 In addition, some studies have shown that RBP secretion is positively regulated by retinol.Citation1
RBP has been used as an index of renal function in clinical practice, as well as to evaluate nutritional status. In two previous studies, RBP was used as a nutritional index for children with acute myeloid leukaemia (AML).Citation5,Citation6 Lower mean RPB levels were observed in paediatric patients with AML than that in healthy controls (n = 52).Citation5 However, no significant difference in RBP level was observed between paediatric patients with AML and those with benign acute diseases.Citation6 Moreover, no differences were observed between paediatric patients with high-risk leukaemia and those at standard risk (n = 173).Citation6 However, thus far, no previous studies have examined RBP levels in adult and elderly patients with AML.
Therefore, the present study was designed to (1) assess RBP levels in AML patients from all age groups and (2) explore potential associations between serum RBP level and clinical characteristics (e.g. white blood cell count and molecular markers) of AML patients.
Methods
Patients and control
A total of 208 patients with de novo AML and 88 patients with benign haematological diseases (e.g. iron deficiency anaemia, aplastic anaemia, and idiopathic thrombocytopenic purpura), as well as 168 healthy subjects, who were all residents of the northeastern region of China (including the Jilin, Hei Longjiang, and Liaoning provinces), were enrolled in this study between 1 January 2006 and 31 December 2011. All of the patients had normal levels of transaminase, albumin, bilirubin, creatinine, and urea nitrogen; those with a previous diagnosis of myelodysplastic syndrome or of a chronic myeloproliferative disorder were excluded from the study. All of the participating patients and healthy subjects provided informed consent prior to enrolment in the study. This study was approved by the Jilin University ethics committee and conducted in accordance with the Declaration of Helsinki.
RBP and prealbumin assay
Peripheral blood samples were collected from patients and healthy subjects, and then RBP (normal range: 30–70 mg/l) and prealbumin (normal range: 0.18–0.39 g/l) concentrations were determined by immunoturbidimetry, using an Automatic Chemistry Analyser (HITACHI 7600, Japan).Citation7 Molar RBP:PA ratio was calculated using the following formula:
Cytogenetic and molecular mutation analyses
Standard cell culturing and chromosome banding techniques were used to analyse karyotypes, and clonal abnormalities were defined and described according to the International System for Human Cytogenetic Nomenclature.Citation8 Mutations of the NPM1 (n = 114), FLT3-ITD (n = 111), and c-kit (n = 87) molecular markers were analysed, and polymerase chain reaction was performed as previously described.Citation9,Citation10 Risk stratification was performed according to the National Comprehensive Cancer Network (NCCN) 2012 guideline.
Statistics
The Statistics Package for Social Sciences (SPSS) software, version 16.0 (SPSS Inc., Chicago, IL, USA) was used to calculate statistical differences. The chi-square test was used to assess the statistical significance of differences between groups. Independent sample t-test or Mann–Whitney U test were used to compare between different groups. Analysis of variance or Kruskal–Wallis H test was used to compare between three or more groups. Correlation analysis was performed using Pearson's or Spearman's correlation test. A P value <0.05 was considered statistically significant.
Results
General characteristics
General characteristics of patients and healthy subjects are summarized in . Patients were categorized according to the French-American-British (FAB) classification system subtypes, based on morphological diagnoses. M2 was the most common subtype in the present cohort (38.46%, n = 80), followed by acute promyelocytic leukaemia (APL; 34.62%, n = 72) and M5 (13.46%, n = 28).
Table 1. General information of the patients and healthy control
RBP concentration
RBP, PA levels, and molar RBP:PA ratios of patients and healthy subjects are presented in . The highest percentage of patients with RBP levels below normal was observed in AML patients, and the lowest percentage was observed in healthy control subjects (A). Conversely, RBP levels were highest in healthy control subjects and lowest in AML patients (B). Non-APL AML patients (32.62 ± 12.94 mg/l) presented with lower RBP concentrations than APL patients (41.17 ± 18.07 mg/l; u = −3.161, P = 0.002).
Figure 1. RBP, PA levels and molar RBP:PA ratio in patients and healthy controls. (A) Percents of patients or healthy subjects whose RBP less than 30 mg/l. (B) RBP levels in AML patients, patients with benign diseases and healthy subjects. (C) PA levels in AML patients, patients with benign diseases and healthy subjects. (D) Molar RBP:PA ratio in AML patients, patients with benign diseases and healthy subjects.
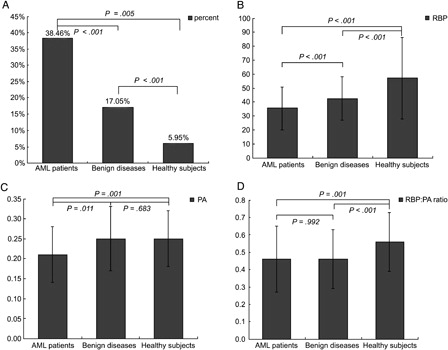
Serum PA levels were significantly lower in AML patients than that in patients with benign haematopathies and normal control subjects (C). Healthy subjects had higher molar RBP:PA ratios than AML patients and patients with benign haematopathies (D).
Correlations between RBP concentration and peripheral white blood cell count, as well as marrow blast
A negative association was observed between serum RBP concentration and peripheral white blood cell (WBC) count (r = −0.179, P = 0.010) across the entire cohort. This negative association was more evident in M4 (r = −0.499, P = 0.036) and M5 (r = −0.412, P = 0.029) patients, but not in other (r = −0.103, P = 0.208) patients, where no significant correlation was observed between RBP concentration and WBC count. Moreover, no association was observed between serum RBP concentration and bone marrow blast percentage specifically among M4 and M5 patients, or across the entire cohort (P > 0.05 for all).
Relationships between RBP concentration and age, gender, FAB classification, cytogenetics, molecular mutations, as well as outcome
Elderly AML patients (aged ≥60 years) had the highest RBP levels compared to paediatric patients (aged ≤16 years; t = 2.284, P = 0.049) and adult patients (aged 16–59 years; t = 2.572, P = 0.011); adult patients had higher RBP levels than paediatric patients (t = 2.076, P = 0.039). However, such differences were not observed in patients with benign diseases (F = 3.851, P = 0.146). In healthy controls, paediatric subjects had lower RPB levels than adult (t = 2.396, P = 0.018) and elderly subjects (u = −3.738, P < 0.001), and no significant difference in RBP levels was observed between adult and elderly subjects (t = 0.137, P = 0.891).
No significant difference in RBP levels were observed between male and female AML patients (male patients: 37.15 ± 15.84 mg/l; female patients: 33.49 ± 14.68 mg/l; t = 1.701, P = 0.09) and patients with benign diseases (male patients: 39.98 ± 15.10 mg/l; female patients: 45.29 ± 15.83 mg/l; t = 1.069, P = 0.111). However, male subjects (62.48 ± 27.43 mg/l) had relatively higher RBP levels than female subjects (52.42 ± 16.76 mg/l) in the healthy control group (t = 2.307, P = 0.023).
The relationships between RBP concentration and FAB subtypes, cytogenetics, as well as molecular markers are listed in . Higher RBP concentrations were observed in APL patients than that in M2 (u = −2.085, P = 0.037), M4 (u = −3.085, P = 0.002), and M5 (u = −2.249, P = 0.024) patients. However, no differences in RBP concentrations were observed when comparing the other subtypes (P > 0.05 for all). Lower RBP concentrations were observed in patients carrying the FLT3-ITD mutation compared to that in those lacking this mutation (u = −2.173, P = 0.030). However, no evident correlations were observed between serum RBP concentration and NPM1 or c-kit mutations.
Table 2. The relationships between FAB subtypes, cytogenetics, and molecular markers and RBP
After achieving complete remission (CR), serum RBP concentrations in AML patients significantly increased from 35.58 ± 15.42 to 47.31 ± 19.50 mg/L (t = 3.953, P < 0.001), which was still lower than the concentrations detected in normal control subjects (t = 2.412, P = 0.017); however, similar molar RBP:PA ratios were observed in AML patients (0.56 ± 0.15) and normal control subjects (0.56 ± 0.17; t = 0.109, P = 0.913). After achieving CR, RBP levels increased by 5.9 (1.30, 15.25) mg/l and 14.9 (1.7, 29.05) mg/L in non-APL and APL patients, respectively. However, no significant difference in the increased RBP level was observed between these two subsets of patients (u = −1.550, P = 0.121). Serum RBP levels at diagnosis were not associated with CR rate after the first cycle of induction therapy (r = −0.061, P = 0.653), relapse (r = −0.073, P = 0.587), or death (r = −0.255, P = 0.219). In addition, RPB levels at remission were not associated with relapse (r = −0.249, P = 0.053) or death (r = −0.183, P = 0.193).
Discussion
To the best of our knowledge, this is the first study evaluating RBP levels in AML patients from all age groups. In this study, the following findings were revealed: (1) AML patients presented with lower RBP levels, especially compared to that in non-APL patients; (2) RBP levels were negatively correlated with peripheral WBC counts in M4 and M5 AML patients; (3) young patients and patients with the FLT3-ITD mutation generally had lower RBP levels than other AML patients; and (4) RBP levels recovered after the patients achieved CR.
RBP serum concentration has been routinely used to evaluate kidney function in clinical practice, and has been widely adopted as a nutritional index.Citation5,Citation6,Citation11 Engle-Stone et al.Citation12 reported that plasma RBP and retinol levels were highly correlated in female subjects (r = 0.94) as well as paediatric subjects (r = 0.96), and some studies have used RBP serum concentration to predict retinol (vitamin A) levels as well.Citation12 In the present study, we excluded subjects with abnormal liver function (may reduce RBP synthesis) and kidney function (may increase serum RBP level) from our analysis, in order to allow a more accurate assessment of RBP level. Consequently, we found that AML patients presented with lower RBP levels than healthy subjects and patients with benign haematological disorders. AML patients also had lower RBP levels than lung cancer patients (see Supplementary material).
Inflammation reduces RBP synthesis in the liver,Citation13 while vitamin A deficiency results in a differential reduction in RBP levels relative to PA, which thereby affects the RBP:PA molar ratio. Since inflammation reduces both RBP and PA levels, it does not result in significant differential reduction in RBP level, and therefore, does not affect the RBP:PA ratio.Citation13 In the present cohort, 47.12% (98/208) of patients presented with inflammatory diseases (e.g. upper respiratory tract infection and pneumonia), and we observed significantly lower RBP levels in these patients (30.07 ± 13.36 mg/L) than that in patients without inflammatory diseases (36.63 ± 13.36 mg/L; t = 2.292, P = 0.024). On the other hand, we observed no significant difference in molar RBP:PA ratios between AML patients with (0.51 ± 0.23) and without (0.45 ± 0.17; t = 1.185, P = 0.24) inflammatory diseases. However, we observed lower molar RBP:PA ratios in AML patients than that in normal control subjects as well as lung cancer patients. These results suggest a decline in retinol levels in de novo AML patients.
WBC count in patients with the FLT3-ITD mutation and in young patients (aged <60 years) was estimated at 42.38 (17.60, 71.50) × 109/l and 8.55 (3.42, 23.97) × 109/l, respectively. These values were significantly higher than that detected in patients lacking the FLT3-ITD mutation (11.45 (4.30, 36.78) × 109/l; u = −2.135, P = 0.033) and in elderly patients (3.80 (1.85, 14.00) × 109/l; u = 2.594, P = 0.018). However, no association was observed between RBP concentration and the FLT3-ITD mutation (r = −0.105, P = 0.287) by partial correlation analysis, after controlling for WBC count. Accordingly, patients with the FLT3-ITD mutation and young patients had lower RBP concentrations that may be associated with high WBC counts. We also established a negative association between RBP level and WBC count (not marrow blasts) in M4 and M5 AML patients. A wealth of evidence revealed by previous studies indicates a crucial role for retinoic acid (RA) during normal myeloid commitment and terminal neutrophil maturation.Citation14 Therefore, one explanation for the association we observed between WBC count and RBP level could be the excessive consumption of RBP during the proliferation of leukaemic cells; however, the underlying mechanisms remain to be determined. After achieving CR, RBP levels in AML patients were lower than those detected in normal control subjects, but similar to those observed in patients with benign haematopathies (47.31 ± 19.50 vs. 42.69 ± 15.62; t = 1.367, P = 0.175). Interestingly, non-APL patients had lower RBP levels than APL patients. However, after achieving CR, increased RBP levels were similar in APL and non-APL patients, despite the fact that all-trans-retinoic acid (ATRA) was administered to APL patients during remission reduction therapy; this observation may suggests the consumption of RA by promyelocytic leukaemic cells.
Controversial results have been reported on the benefits of ATRA therapy for AML (not APL) patients. Some studies observed no benefits for using ATRA in induction therapy.Citation15,Citation16 However, Schlenk et al.Citation17 reported that the addition of ATRA to induction and consolidation therapy improved CR, event-free survival, and overall survival rates in elderly AML patients. Each of these studies used a different course for the ATRA treatment, which may explain the differences in results.Citation15 Accordingly, APL patients and non-APL elderly patients can benefit from ATRA therapy. Interestingly, both of these patient subgroups had higher RBP levels than other AML patients in our study. As mentioned above, after achieving CR, the increased RBP levels in APL (although ATRA was used) and non-APL patients were similar. This may indicate that the high RBP levels (possibly retinol levels as well) in elderly patients enhanced the benefits of ATRA therapy, and consumption of ATRA in APL patients resulted in achieving similar recovery of RBP levels as that observed non-APL patients. Moreover, all these findings indicated that insufficient administration of ATRA may have had no beneficial effect on non-APL AML patients. This study's limitation was that we did not detect corresponding retinol or vitamin A levels; we plan to examine that in future studies. However, it is RBP that carries retinol in the blood and transports it to target cells via the RBP receptor. Therefore, as the specific carrier of retinol, RBP may serve as more than a nutritional index. In conclusion, these findings suggest that aberrant retinol levels may be associated with AML.
Acknowledgements
We thank the Cancer Center at the First Hospital, Bethune Medical College of Jilin University, for their assistance in this work.
References
- Raghu P, Sivakumar B. Interactions amongst plasma retinol-binding protein, transthyretin and their ligands: implications in vitamin A homeostasis and transthyretin amyloidosis. Biochim Biophys Acta. 2004;1703:1–9.
- Newcomer ME, Ong DE. Plasma retinol binding protein: structure and function of the prototypic lipocalin. Biochim Biophys Acta. 2000;1482:57–64.
- Moise AR, Noy N, Palczewski K, Blaner WS. Delivery of retinoid-based therapies to target tissues. Biochemistry. 2007;46:4449–58.
- Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochem J. 2000;348:481–95.
- Kuvibidila S, Yu L, Gardner R, Velez M, Ode D, Warrier RP. Association between increased levels of TNF-alpha, decreased levels of prealbumin and retinol-binding protein, and disease outcome. J Natl Med Assoc. 2000;92:485–91.
- Uderzo C, Rovelli A, Bonomi M, Barzaghi A, Strada S, Balduzzi A, et al. Nutritional status in untreated children with acute leukemia as compared with children without malignancy. J Pediatr Gastroenterol Nutr. 1996;23:34–7.
- Bankson DD, Rifai N, Silverman LM. Immunoturbidimetric measurement of serum retinol-binding protein in renal and hepatic disease. Ann Clin Biochem. 1988;25:246–9.
- Shaffer LG, Slovak ML, Campbell LJ, editors. An international system for human cytogenetic nomenclature, ISCN. Basel: S. Krager; 2009.
- Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18.
- Rulina AV, Spirin PV, Prassolov VS. Activated leukemic oncogenes AML1-ETO and c-kit: role in development of acute myeloid leukemia and current approaches for their inhibition. Biochemistry (Mosc). 2010;75:1650–66.
- Drescher T, Singler K, Ulrich A, Koller M, Keller U, Christ-Crain M, et al. Comparison of two malnutrition risk screening methods (MNA and NRS 2002) and their association with markers of protein malnutrition in geriatric hospitalized patients. Eur J Clin Nutr. 2010;64:887–93.
- Engle-Stone R, Haskell MJ, Ndjebayi AO, Nankap M, Erhardt JG, Gimou MM, et al. Plasma retinol-binding protein predicts plasma retinol concentration in both infected and uninfected Cameroonian women and children. J Nutr. 2011;141:2233–41.
- Rosales FJ, Ross AC. A low molar ratio of retinol binding protein to transthyretin indicates vitamin A deficiency during inflammation: studies in rats and a posterior analysis of vitamin A-supplemented children with measles. J Nutr. 1998;128:1681–7.
- Lawson ND, Berliner N. Neutrophil maturation and the role of retinoic acid. Exp Hematol. 1999;27:1355–67.
- Burnett AK, Hills RK, Milligan DW, Goldstone AH, Prentice AG, McMullin MF, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2010;28:586–95.
- Estey EH, Thall PF, Pierce S, Cortes J, Beran M, Kantarjian H, et al. Randomized phase II study of fludarabine+cytosine arabinoside+idarubicin +/− all-trans retinoic acid +/− granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93:2478–84.
- Schlenk RF, Fröhling S, Hartmann F, Fischer JT, Glasmacher A, del Valle F, et al. Phase III study of all-trans retinoic acid in previously untreated patients 61 years or older with acute myeloid leukemia. Leukemia. 2004;18:1798–1803.