Abstract
Background
Sickle cell disease (SCD) is a chronic, incurable hereditary disease. The vaso-occlusive crisis (VOC) is the most frequently occurring acute complication in sickle cell patients and accounts for the majority of SCD-related hospital admissions. Another major complication is the potentially fatal acute chest syndrome (ACS). The prototypic long pentraxin-3 (PTX3), an acute phase protein and a key component of innate immunity, is linked to ischemia-induced inflammation, a condition incriminated in SCD complications.
Aim
To investigate the expression of PTX3 in stable SCD and VOC patients and to assess its relation to the development and progression of ACS.
Subjects and methods
We conducted this study on 160 patients with confirmed SCD (20 stable SCD and 140 in VOC), and 10 healthy age- and sex-matched controls. Patients were diagnosed as SCD by high-performance liquid chromatography. PTX3 levels were assessed using enzyme-linked immunosorbant assay.
Results
In the stable state, all 20 SCD patients had PTX3 levels (range = 0.9–2.1 ng/ml; median = 1.1) comparable to those of healthy controls (range = 0.8–2.0 ng/ml; median = 1.0) (P > 0.05). During the VOC, plasma PTX3 significantly increased (range = 8.7–37.2 ng/ml; median = 22.3) (P < 0.01). Out of 140 VOC patients, 15 (10.7%) developed ACS and four required mechanical ventilation, of which two died. The median plasma level of PTX3 (22.3 ng/ml) was set as a cut-off value to stratify patients into low- and high-PTX3 expressers. Of the 140 VOC patients, 43 (30.7%) had PTX3 levels >22.3 ng/ml, of these, 13 patients developed ACS (13/43; 30.2%); of the remaining 97 patients who had PTX3 ≤22.3 ng/ml, only two patients (2/97; 2.1%) progressed to ACS, with a further increment in PTX3 in all of them. PTX3 levels were correlated with length of hospital stay in VOC patients and markers of lung injury in ACS patients.
Conclusion
PTX3 levels were higher in SCD patients in VOC, being associated with longer hospital stay. Higher initial PTX3 concentrations were related to the development of ACS with a further increase in PTX3 levels observed upon progression to ACS. Thus, PTX3 could be used as a subjective method to predict occurrence and severity of SCD acute complications.
Introduction
Sickle cell disease (SCD) is a chronic, incurable hereditary disease presenting primarily as anemia (sickle cell anemia) in people who are homozygous for hemoglobin S (HbS). This abnormal hemoglobin (Hb), resulting from the replacement of glutamic acid at position 6 of the β-globin chain by valine, is responsible for erythrocyte distortion and fragility.Citation1 Information about the prevalence of SCD in Saudi Arabia is patchy, probably underestimated, and varies significantly in different parts of the country, with the highest being in the eastern province, followed by the south-western provinces.Citation2 Five different DNA mutations have been identified for the sickle cell gene worldwide, of which eastern province patients' population represents an important entity of its own since these patients have a different clinical course.Citation3
The painful or vaso-occlusive crisis (VOC) is the most frequently occurring acute complication in sickle cell patients and accounts for the majority of SCD-related hospital admissions.Citation4,Citation5 It presents as acute musculoskeletal and/or visceral pain which often requires hospital admission for intravenous hydration and opiates.Citation4 The hallmark of this condition is vaso occlusion that occurs in almost all vascular beds leading to ischemic tissue injury with organ dysfunction and early death. Outcome is difficult to predict, and few effective therapeutics are available.Citation6 Clinical complications of SCD are vast, from simple painful episodes to stroke, acute chest syndrome (ACS), or bone infarcts.
The sequence of events that lead to the sickle cell VOC is not well understood. Several authors have outlined an order of events occurring in the microcirculation that ends in this painful sickle cell crisis; namely, polymerization of HbS, decreased blood red cell deformability, microvascular occlusion, and hypoxia of tissues involved with the subsequent painful irritation of adjacent nerve endings.Citation7–Citation10 Ischemic events produced by the occlusion of both large and small blood vessels are stressful and involve multiple interactions between red blood cells, the endothelium, and leukocytes.Citation11,Citation12 These interactions are known to be regulated by cytokines secreted by T cells as well as by adhesion molecules, and consequently, the immune response is implicated in the initiation and development of the sickle cell VOC.Citation1 Several inflammatory biomarkers have been investigated during the course of VOC, including interleukins (ILs) IL-2, IL-4, IL-10,Citation1 and IL-812 as well as high-sensitivity C-reactive protein (CRP),Citation13 and heat shock protein-70.Citation14 However, up-to-date, no established objective laboratory tools to confirm the diagnosis and to assess the severity of the VOC exist, which renders its management one of the most challenging aspects of caring for sickle cell patients. Moreover, in ACS, phospholipase A2 has been proposed as a promising positive predictive marker;Citation15 though more recent studies have reported a lower predictive impact.Citation16,Citation17
The prototypic long pentraxin-3 (PTX3) is an acute phase protein and a key component of innate immunity. In contrast to CRP, which is produced mainly in the liver in response to pro-inflammatory stimuli, PTX3 is produced and released locally at the site of inflammation by several cell types, including mononuclear phagocytes, endothelial cells, dendritic cells, and fibroblasts. Furthermore, PTX3 can activate endothelial cells and up-regulate tissue factor expression by endothelial cells and monocytes, thus can potentially induce both endothelial and coagulation activation.Citation18,Citation19 PTX3 levels were found to be high in patients experiencing ischemia/tissue infarcts, like myocardial infarction (MI) patients;Citation19,Citation20 therefore, it is of rationale to implicate PTX3 in SCD-induced complications, where the development of microinfarcts seems to be the main underlying pathophysiological event.
In our work, we tested the hypothesis that PTX3 is a good predictive tool for SCD acute complications, namely VOC and ACS. Whether or not PTX3 expression is linked to SCD Hb subtypes was also tested.
Subjects and methods
Between June 2011 and May 2012, 140 consecutive SCD patients (≥16 years old), from two medical institutes in the eastern region of Saudi Arabia (The Royal Commission Hospital and Naval Base Hospital) who presented with VOC, defined as an attack of severe musculoskeletal pain not responding to regular analgesics and cannot be otherwise attributed to any other diagnosis, were included. Twenty SCD patients in stable state, defined as having no acute SCD-related complications as VOC, priapism, or stroke, were also included. Ten healthy age- and sex-matched subjects served as a control group. All enrolled patients and healthy subjects gave written informed consent. This study was reviewed and accepted by the ethical and research committee of both institutes and in accordance with the Helsinki declaration of 2000. The management protocols for VOC and ACS, as well as indication for blood transfusion were similar in both institutes, and in line with the international published data.Citation21,Citation22
All patients had a full clinical examination after a thorough medical history. Patients with definite inflammation and/or infection were excluded. Pregnant females and patients who received blood in the previous 120 days were also excluded from this study.
Blood samples were obtained during the routine follow-up in the outpatient clinic for stable SCD, or at presentation in emergency room for VOC patients (sample 1). VOC patients were managed according to the protocols of VOC management, and those who responded to treatment were discharged. For those patients who progressed to ACS while in the hospital, defined as a new infiltrate on chest radiograph in conjunction with one other new symptom or sign: chest pain, cough, wheezing, tachypnea, and/or fever (> 38.5°C), once the diagnosis was established, a second time sample (sample 2) was obtained before further clinical intervention. No further samples were included in this study due to blood transfusion used as part of management protocol for ACS.Citation22
Clotted samples were used for the determination of serum lactate dehydrogenase, total bilirubin, and CRP levels (Vitros, 5.1; Ortho Clinical Diagnostics, Inc., Buckinghamshire, UK).
EDTA anti-coagulated samples were used for standard blood count (Cell-Dyn Sapphire; Abbot Laboratories, IL, USA) and for the confirmation of diagnosis of SCD and its sub-classification into HbSS, HbSβ0, and HbSHPFH by high-performance liquid chromatography (Variant II, Biorad, CA, USA).
For the determination of plasma PTX3 concentration, EDTA anti-coagulated samples were centrifuged at 1000 g for 15 minutes, within 30 minutes from collection, and the plasma stored at −80°C for further analysis using sandwich-type enzyme-linked immunosorbant assay (Human PTX3 Quantikine, R&D systems, Minneapolis, MN, USA).
Statistical analysis
Quantitative data were expressed in the form of range, mean ± SD, or median and were compared with Student's t test when parametric and Wilcoxon-Rank-sum test when non-parametric. Qualitative data were expressed in the form of number and percent and comparisons were made by χ2 or Fisher's exact test wherever appropriate. Correlations were made by Spearman's rank correlation coefficient in cases of non-parametric variables and Pearson's correlation for parametric variables. All tests being two-tailed. A P value <0.05 was considered statistically significant. Analysis was made by Graphpad prism software version 5.0 (Graphpad software Inc., La Jolla, CA, USA).
Results
This cross-sectional observational study was carried out on 140 consecutive SCD patients with VOC, 20 SCD patients in stable state, and 10 healthy age- and sex-matched control subjects during the period from June 2011 to May 2012.
Of the 140 SCD patients with VOC, 125 had non-complicated VOC, while 15 patients (10.7%) were subsequently complicated by ACS; of these 15 patients, four patients (26.7%) developed clinical manifestations of respiratory failure (anxiety, tachypnea, tachycardia, diaphoresis, arrhythmias, hyper- or hypotension, altered mental state, confusion, and cyanosis); blood gases analysis revealed hypoxia (partial pressure of oxygen in blood (PaO2) <60 mmHg) and hypercapnea (partial pressure of carbon dioxide in blood >50 mmHg) in the absence of compensation by metabolic alkalosis; the ratio of PaO2 to the fraction of inspired O2 (FiO2) (PaO2/FiO2) dropped to <200 and mechanical ventilation was needed. Two of the four ventilated ACS patients (50%) survived and two died.
Of the 20 patients who were in stable condition, with no evidence of sickle cell-related acute complications, 12 patients (60%) had HbSS, three (15%) had HbSβ0, and five (25%) had HbSHPFH. Of the 140 patients who were admitted with VOC, 84 patients (60%) had HbSS, 19 (13.6%) had HbSβ0, and 37 (26.4%) had HbSHPFH; of these, 15 patients (10.7%) were complicated by ACS all of them were HbSS. Clinical and laboratory data of the subjects included in this study are summarized in .
Table 1. Clinical and laboratory data
PTX3 and CRP levels in stable SCD and VOC patients
In the stable state, all 20 SCD patients had PTX3 levels (range = 0.9–2.1 ng/ml; median = 1.1) comparable to those of healthy controls (range = 0.8–2.0 ng/ml; median = 1.0) (P > 0.05). During the VOC, plasma PTX3 significantly increased (range = 8.7–37.2 ng/ml; median = 22.3) (P < 0.01) (A), not being correlated to any of the laboratory studied parameters, including markers of hemolysis (total bilirubin and lactate dehydrogenase) or CRP, but significantly positively correlated with length of hospital stay (P < 0.01) (). In an attempt to fully analyse the relation of plasma PTX3 concentration at presentation to the length of hospital stay, the correlation studies were performed in VOC patients both including and excluding ACS sample 1 PTX3 results in order to eliminate the effect of the more prolonged hospitalization time observed in all ACS patients. PTX3 level proved to be significantly associated with longer hospital stay whether on considering patients with VOC only (hospital stay = 3–19 days; median = 10) (rs = 0.35; P = 0.01) or on additional inclusion of those who progressed to ACS (hospital stay = 3–24 days; median = 15) (rs = 0.38; P < 0.01) ().
Figure 1. (A) Levels of PTX3 are comparable in normal controls and stable SCD patients, whereas they are significantly increased in VOC (P < 0.01). (B) Levels of CRP are increased in stable SCD compared to controls (P < 0.01), with a further increment in VOC (P < 0.01). The box and whisker plots show in the box the median and the 25th and 75th percentile; whiskers show the 2.5th and 97.5th percentile.
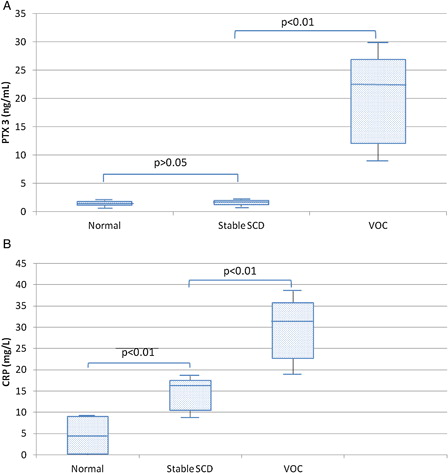
On the other hand, CRP levels were significantly higher in stable SCD patients (range = 8.6–18.7 mg/L; median = 16.1) than in controls (range = 0–9.2 mg/L; median = 4.6) (P < 0.01), with a further increase in VOC patients (range = 17.2–40.9 mg/L; median = 31.4) (P < 0.01) (B). CRP levels showed no correlation with any of the studied laboratory parameters or the length of hospital stay (data not shown).
Relation of PTX3 and CRP levels to development of ACS
The median plasma level of PTX3 (22.3 ng/ml) was set as a cut-off value to stratify patients into low- (≤22.3 ng/ml) and high-PTX3 (>22.3 ng/ml) expressers. Forty-three of the 140 stable patients (30.7%) were high-PTX3 expressers; of these, 13 patients developed ACS (13/43; 30.2%), while out of the remaining 97 patients who were low-PTX3 expressers, only two (2/97; 2.1%) progressed to ACS; the comparison being statistically significant (P < 0.01) ().
Table 2. Relation of PTX3 and CRP levels to development of ACS and Hb variant
As for CRP, the median value (31.4 mg/l) was used as a cut-off value, and 63/140 patients (45%) had higher CRP levels (>31.4 mg/l), while 77 (55%) had levels ≤31.4 mg/l. However, 8/63 (12.7%) patients with higher CRP levels and 7/77 (9%) patients with lower CRP developed ACS, the comparison being statistically insignificant (P > 0.05) ().
Of note, although all 15 patients who developed ACS were HbSS, higher PTX3 levels were not significantly related to the type of Hb variant (HbSS, HbSβ0, or HbSHPFH), as was the case with higher CRP levels (P > 0.05) ().
PTX3 and CRP levels in ACS
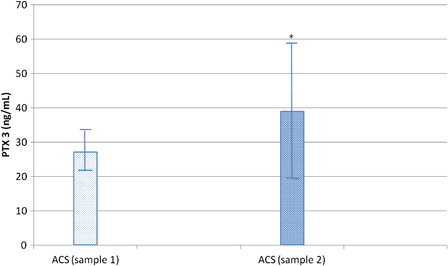
Second time measurement of PTX3 level after the establishment of diagnosis of ACS and before the start of blood transfusion (sample 2) revealed further significant increment of PTX3 concentration (range = 23.9–88.4 ng/ml; mean ± SD = 39.03 ± 19.45) over its level in sample 1 (range = 19.7–37.2; mean ± SD = 27.03 ± 5.323) (P < 0.01) (). On the contrary, no significant difference in CRP levels (34.6 ± 12.9 vs. 31.3 ± 13.2) (P > 0.05) was observed between samples 2 and 1 of ACS patients.
Figure 2. PTX3 levels are significantly increased in the second sample of patients who developed ACS compared to their first sample at the time of VOC diagnosis (*P < 0.01 compared to sample 1). Bars represent mean ± SD.
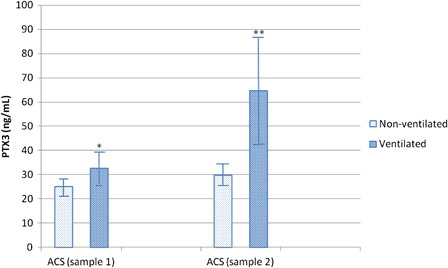
On comparing the mean plasma PTX3 levels in non-ventilated (n = 11) and ventilated (n = 4) ACS patients in samples 1 and 2, PTX3 concentrations were significantly higher in ventilated patients in both occasions: ACS sample 1 ventilated patients PTX3 mean ± SD 32.33 ± 6.5 vs. 25.09 ± 3.3 in non-ventilated patients (P = 0.01) and ACS sample 2 ventilated PTX3 mean ± SD 64.75 ± 22.63 vs. 29.69 ± 4.7 in non-ventilated patients (P < 0.001) ().
Figure 3. PTX3 level was significantly increased in ventilated ACS patients in sample 1 (*P = 0.01 compared to non-ventilated patients) and sample 2 (**P = 0.0002 compared to non-ventilated patients). Bars represent mean ± SD.
The higher PTX3 observed in ACS sample 2 patients significantly correlated with lower PaO2/FiO2, taken as a marker of lung injury and an indicator of mechanical ventilation (r = −0.84; P < 0.01) (). Moreover, on correlating ACS patients' PTX3 level at presentation (sample 1) with the PaO2/FiO2, measured later on during the course of illness, a significant negative correlation existed (r = −0.73; P < 0.01). Plasma PTX3 levels in ACS patients did not significantly correlate with any of the studied laboratory parameters, including CRP. Also, no statistical correlation could be established between PTX3 concentrations in ACS patients and length of hospital stay (14–24 days; median = 19) ().
Table 3. Correlation of PTX3 level to laboratory data, PaO2/FiO2, and hospital stay in stable SCD, VOC, and ACS patients
Of note, sample 2 plasma PTX3 levels in the two expired patients (65.1 and 88.4; mean ± SD = 76.75 ± 16.47) were higher than the levels in the two survivors (34.1 and 71.3; mean ± SD = 52.7 ± 26.33), though not reaching statistical significance (P > 0.05).
Discussion
The lack of an objective tool to assess the presence or absence of VOC and to early predict the dreadful complication of ACS remains to be a strong driving force for researchers to find a simple laboratory tool capable of predicting the outcome of SCD patients. Since PTX3 is a well-studied marker in relation to inflammation, infection, and ischemia,Citation20,Citation23 we have decided to explore its relationship with VOC and ACS.
Our results show that PTX3 level in asymptomatic SCD patients were low (being almost normal) in comparison to patients experiencing VOC, and subsequently higher in patients who progressed to ACS. On the other hand, CRP levels were high in patients with SCD as a baseline, with a further increase in VOC, showing no significant correlation either with hospital stay, or progression to ACS. This can be explained by the fact that despite the presence of a background state of chronic inflammation manifested as high baseline CRP values, an actual vascular occlusion and subsequent tissue ischemia (like that encountered in VOC) with the resultant apoptotic or necrotic cells, is needed to trigger a rise in PTX3 level derived from activated neutrophils and endothelial cells, which in turn regulates the complement binding to dead cells and their uptake by phagocytosis as part of its role in innate immunity.Citation24 Here, it is important to note that due to the documented relation of PTX3 to infection,Citation24,Citation25 we have exerted every possible effort to exclude patients with documented infection as a triggering factor for their VOC or ACS, as well as those with positive bacterial cultures in an attempt to scope those patients with a predominantly vascular etiology rather than an infective one.
Furthermore, contrary to CRP, higher PTX3 levels at presentation were significantly associated with longer hospital stay in VOC patients. However, no such association could be established in ACS patients since their number was relatively small and all of them had comparably prolonged hospital stays.
Nur et al.Citation26 have previously demonstrated the variation in PTX3 concentrations during the clinical course of SCD, with increased levels during VOC, though with much lower values compared to those reported in the study at hand; a difference attributable to one or all of the following factors; namely, the relatively smaller sample size of VOC patients (33 cases) included in the Nur et al.Citation26 study, together with the fact that this study included 15 ACS patients with progressive clinical course and very high PTX3 levels, and finally the significantly different Hb variant subtypes included in both studies with the documented unique nature, clinical course, and hybrid Hb variants found in eastern province of Saudi Arabia.Citation2,Citation3 To our knowledge, this is the first report describing levels of PTX3 as well as rates of ACS in SCD patients among Saudi population. Our results showed no difference in PTX3 level based on HbS percentage, or the presence of other Hb variants. Also, our ACS population was relatively small (15/140; 10.7%) with a considerably lower death rate of 2/15 (13%) compared to the previously reported rates approaching 25%.Citation27,Citation28 Therefore, we believe that these results need larger sample size studies for better evaluation and validation.
For retrospective evaluation, patients were stratified according to PTX3 expression taking the median as a cut-off level (22.3 ng/ml), which revealed that patients with lower PTX3 levels had a significantly less probability to progress to ACS (2/97; 2.1%) compared to those with higher PTX3 levels (13/43; 30.2%) (P < 0.01). Moreover, development of ACS was accompanied by a further significant increment in plasma PTX3 concentration noted in a second sample obtained after the diagnosis was established and before any further management, probably due to the ability of human lung epithelial cells to produce PTX3 upon pro-inflammatory stimulation.Citation29 No such findings could be established in CRP levels, as it is produced systemically in the liver thus requiring longer time to reach peak levels. This local vs. systemic site of production in PTX3 and CRP could account for the lack of relation between their levels observed in the study at hand, as well as the earlier rise of PTX3 noted in SCD and other pathological conditions, such as MI.Citation19,Citation20
Additionally, PTX3 levels were significantly higher in patients who later needed mechanical ventilation in both first and second samples, being significantly negatively correlated with PaO2/FiO2, taken as a marker of lung injury and an indication for mechanical ventilation. It is noteworthy that the negative correlation of PTX3 level at presentation with the later measured marker of lung injury (PaO2/FiO2) signifies that PTX3 level could serve as a possible early predictor of ACS patients deterioration. Our findings are in line with those of Mauri et al.,Citation30 who demonstrated that PTX3 level was elevated in acute lung injury and acute respiratory distress syndrome and its high levels positively correlated with parameters of lung injury and systemic involvement. These data suggest that plasma PTX3 level, can predict not only the occurrence of the life-threatening complication, ACS, in SCD-induced VOC, but can also serve as an early indicator of its severity. More studies are needed to establish such promising observations. However, available supporting evidence exist; CRP levels in VOC were shown to reach highest values in 72 hours from hospital admissionCitation31 and 50 hours from the onset of symptoms in MI patients, whereas PTX3 concentrations reached their peaks in just 7 hours from the onset of symptoms, returning towards baseline in a few days;Citation19 suggesting the value of PTX3 as an early marker of ischemia-induced inflammation.Citation20,Citation24 In our work, we measured PTX3 levels once only at presentation in all candidates except those patients who progressed to ACS where a second sample was taken; further studies utilizing repeated sampling to outline the dynamics of PTX3 secretion in ACS are required, though the use of blood transfusion as part of the management is a major hindrance.
It is noteworthy that the increase of PTX3 level in VOC or ACS may serve as a protective tool against inflammation-associated tissue damage, through a negative feedback action on P-selectin to restrict neutrophil migration into tissuesCitation25 together with inhibition of unwanted complement activation in circulating blood through binding of fluid-phase PTX3 to free Cq1,Citation32 in an attempt to dampen unwanted dissemination of inflammatory reactions. In fact, it has been shown that lack of PTX3 can worsen lipopolysaccharide-induced acute lung injury through enhancement of cell necrosis, neutrophil recruitment, activation of coagulation cascades, and inflammatory responses;Citation33 while the in vivo injection of PTX3 is capable of reducing the number of neutrophils rolling in thrombin-stimulated mesenteric venules of mice.Citation24
In conclusion, our data suggest that plasma concentration of PTX3 in SCD patients experiencing VOC is a potential, promising early prognostic biomarker capable of identifying patients more prone to complications allowing for their prompt and more radical clinical management, which may warrant changes to the current treatment modalities giving space to early blood transfusion either simple or in the context of exchange transfusion aiming at a better outcome and shortened hospital stay. We believe that this study is the first in our region to tackle this point, yet further larger number multicenter trials are well needed to validate our findings, and to establish cut-off and predictive values for the clinical utilization of PTX3 levels.
References
- Musa BO, Onyemelukwe GC, Hambolu JO, Mamman AI, Isa AH. Pattern of serum cytokine expression and T-cell subsets in sickle cell disease patients in vaso-occlusive crisis. Clin Vaccine Immunol. 2010;17(4):602–8.
- Jastaniah W. Epidemiology of sickle cell disease in Saudi Arabia. Ann Saudi Med. 2011;31(3):289–93.
- Alabdulaali MK. Sickle cell disease patients in eastern province of Saudi Arabia suffer less severe acute chest syndrome than patients with African haplotypes. Ann Thorac Med. 2007;2:158–62.
- Serjeant GR, Ceulaer CD, Lethbridge R, Morris J, Singhal A, Thomas PW. The painful crisis of homozygous sickle cell disease: clinical features. Br J Haematol. 1994;87(3):586–91.
- Platt OS, Harington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, et al. Pain in sickle cell disease. Rates and risk factors.N Engl J Med. 1991;325(1):11–6.
- Baum KF, Dunn DT, Maude GH, Serjeant GR. The painful crisis of homozygous sickle cell disease: clinical features. Br J Haematol. 1994;87(3):586–91.
- Francis RB, Johnson CS. Vascular occlusion in sickle cell disease: current concepts and unanswered questions. Blood. 1991;77(7):1405–14.
- Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle cell disease pathophysiology. Blood. 1991;77(2):214–37.
- Platt OS. Easing the suffering caused by sickle cell disease. N Engl J Med. 1994;330(11):783–4.
- Powars DR. Sickle cell anemia and major organ failure. Hemoglobin. 1990;14(6):573–98.
- Ballas SK, Mohandas N. Pathophysiology of vaso-occlusion. Hematol Oncol North Am. 1996;10(6):1221–39.
- Duits AJ, Schnog JB, Lard LR, Saleh AW, Rojer RA. Elevated IL-8 levels during sickle cell crisis. Eur J Haematol. 1998;61(5):302–5.
- Krishnam S, Setty Y, Betal SG, Vijender V, Rao K, Dampier C, et al. Increased levels of the inflammatory biomarker C-reactive protein at baseline are associated with childhood sickle cell vaso-occlusive crisis. Br J Haematol. 2010;148(5):797–804.
- Adewoye AH, Klings ES, Farber HW, Palaima E, Bausero MA, McMahon L, et al. Sickle cell vaso-occlusive crisis induces the release of serum heat shock protein-70. Am J Hematol. 2005;78(3):240–2.
- Styles LA, Aarsman AJ, Vickinsky EP, Kuypres FA. Secretory phospholipase A2 predicts impending acute chest syndrome in sickle cell disease. Blood. 2000;96(9):3276–8.
- Ballas SK, Files B, Jones LL, Benjamin L, Swerdlow P, Hilliard L, et al. Secretory phospholipase A2 levels in sickle cell disease and acute chest syndrome. Hemoglobin. 2006;30(2):165–70.
- Styles LA, Wager CG, Labotka RJ, Smith-Whitley K, Thompson A, Lane PA, et al. Refining the value of secretory phospholipase A2 as a predictor of acute chest syndrome in sickle cell disease: results of a feasibility study (PROACTIVE). Br J Haematol. 2012;157(5):627–36.
- Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28(1):1–13.
- Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, et al. PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102(6):636–41.
- Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110(16):2349–54.
- Rezaei-Kalantari H. How I treat … sickle cell anemia: current therapies. Rev Med Liege. 2001;56(10):671–5.
- Lottenberg R, Hassell KL. An evidence-based approach to the treatment of adults with sickle cell disease. ASH Education Book 2005;1:58–65.
- Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29(7):1404–7.
- Kunes P, Holubcova Z, Kolackova M, Krejeski J. Pentraxin 3: an endogenous modulator of the inflammatory response. Mediators Inflamm. 2012; 2012(article ID 920517): 10.
- Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leucocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11(4):328–34.
- Nur E, van Beers EJ, Martina S, Cuccovillo I, Otten HM, Schnog JJ, et al. Plasma levels of pentraxin-3, an acute phase protein, are increased during sickle cell painful crisis. Blood Cells Mol Dis. 2011;46(3):189–94.
- Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B. Acute chest syndrome in sickle cell disease: clinical presentation and course. Cooperative study of sickle cell disease. Blood. 1997;89(5):1787–92.
- Miller ST, Sleeper LA, Pegelow CH, Enos LE, Wang WC, Weiner SJ, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342(2):83–9.
- Han B, Mura M, Andrade CF, Okutani D, Lodyga M, dos Santos CC, et al. TNFα-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J Immunol. 2005;175:8303–11.
- Mauri T, Coppadoro A, Bellani G, Bambino M, Patroniti N, Peri G, et al. Pentraxin 3 in acute respiratory distress syndrome: an early marker of severity. Crit Care Med. 2008;36(8):2302–8.
- Bargoma EM, Mitsuyoshi JK, Larkin SK, Styles LA, Kuypers FA, Test ST. Serum C-reactive protein parallels secretory phospholipase A2 in sickle cell disease patients with vaso-occlusive crisis or acute chest syndrome. Blood. 2005;105:3384–5.
- Nauta AJ, Bottazzi B, Mantovani A. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol. 2003;33(2):465–73.
- Han B, Haitsma JJ, Zhang Y, Bai X, Rubacha M, Keshavjee S, et al. Long pentraxin PTX3 deficiency worsens LPS-induced acute lung injury. Intensive Care Med. 2011;37:334–42.
