Abstract
The D-index is calculated as the area over the neutrophil curve during neutropenia. We investigated the impact of the D-index on pulmonary infection in 33 acute myeloid leukemia patients undergoing consolidation chemotherapy with high-dose cytarabine. There was no difference in the D-index between chemotherapies with and without pulmonary infection. The cumulative D-index (c-D-index) until the development of infection exceeded 4000 in four of five patients with pulmonary infection. Although there was no difference in the total D-index throughout the overall consolidation chemotherapy, the total D-index from induction to consolidation and the D-index at induction chemotherapy were higher in patients with pulmonary infection during consolidation than in those without it (P = 0.014 and 0.019, respectively). Our results showed that the cumulative effect of neutropenia might determine the risk of pulmonary infection in consolidation chemotherapy. We are planning a clinical trial of c-D-index-guided preemptive antifungal therapy.
Introduction
Neutropenia is a major risk factor for infections in leukemia patients undergoing intensive chemotherapy.Citation1 The D-index was first proposed by Portugal et al.Citation2 as a tool for evaluating the dynamics of neutropenia. It was based on a graph that showed the absolute neutrophil count during neutropenia (<500/μl) and was calculated as the area over the neutrophil curve. Thus, it could be used to evaluate both the intensity and duration of neutropenia. A cumulative D-index (c-D-index), defined as the cumulative D-index from the start of neutropenia until the development of infection, could be used for the real-time assessment of the risk for infection. The c-D-index has been shown to have a high negative predictive value for invasive mold infection (IMI) in acute myeloid leukemia (AML) patients undergoing induction chemotherapyCitation2 and for pulmonary infection in recipients of hematopoietic stem cell transplantation (HSCT).Citation3
High-dose cytarabine (HDAC) is widely used as an intensive consolidation chemotherapy regimen in adult AML patients.Citation4,Citation5 Although the duration of neutropenia in each consolidation cycle is generally shorter, and therefore the risk of infection is lower than in induction chemotherapy, infectious complications are still a problematic issue.6–10 However, it is not yet clear whether the D-index is also useful for predicting infection during consolidation chemotherapy. In addition, the impact of neutropenia in previous courses of chemotherapy on infection during consolidation chemotherapy has not been well defined. In this study, we retrospectively investigated the impact of the D-index on pulmonary infection in AML patients in first complete remission (CR1) undergoing consolidation chemotherapy with HDAC.
Methods
Patients and treatment procedure
AML patients who achieved CR1 by one or two induction chemotherapies and received HDAC as consolidation therapy in our medical center between March 2007 and July 2011 were consecutively enrolled in this retrospective study. This study was approved by the Institutional Review Board of Saitama Medical Center, Jichi Medical University. Thirty-three patients were included in this study.
As the first induction chemotherapy, all patients were treated with idarubicin 12 mg/m2 daily by intravenous infusion over 30 minutes on days 1–3 and with cytarabine 100 mg/m2 daily by continuous intravenous infusion on days 1–7 (IDA-AraC). Five patients who did not achieve complete remission received a second induction chemotherapy, which consisted of IDA-AraC (n = 1) or a combination of cytarabine 2 g/m2 by intravenous infusion over 3 hours at 12-hour intervals on days 1–4 and mitoxantrone 7 mg/m2 daily by intravenous infusion over 30 minutes on days 1 and 2 (n = 4).
Consolidation chemotherapy consisted of cytarabine 2 g/m2 by intravenous infusion over 3 hours at 12-hour intervals on days 1–5. In patients over 60 years old, the dose of cytarabine was reduced to 1.5 g/m2 (n = 2). Eighteen patients completed three courses of HDAC. The others did not because of transition to stem cell transplantation in CR1 (n = 5), severe side effects (n = 3), and relapse during consolidation (n = 7). Granulocyte colony-stimulating factor was administered in only four chemotherapy cycles after the development of documented infection. Prophylaxis against bacterial and fungal infections was performed in all patients. The former consisted of fluoroquinolone, mostly levofloxacin. The latter consisted of fluconazole (n = 3), itraconazole (n = 26), and other anti-fungal agents (n = 4). Topical corticosteroid and eye rinse with sterile saline were used to prevent cytarabine-induced kerato-conjunctivitis.
Neutropenia indexes and pulmonary infection
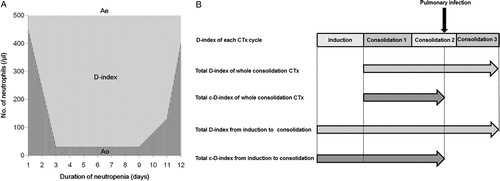
The D-index of each chemotherapy cycle was calculated based on a graph that plotted the absolute neutrophil counts over the course of the episode of neutropenia.Citation2 The D-index was calculated as the difference between the observed area under the curve, which was calculated by the trapezoidal method, and the expected neutrophil area (500/μl x days with neutropenia) if the patient did not develop neutropenia (A). The c-D-index was calculated as the cumulative D-index from the start of neutropenia until the development of infection in patients with pulmonary infection, whereas the c-D-index was equal to the D-index in patients without infection. We also evaluated the duration of neutropenia (<500/μl) and profound neutropenia (<100/μl) in each chemotherapy cycle. To investigate the impact of the cumulative effect of neutropenia including previous chemotherapies, we calculated total neutropenia indexes as shown in B; the total D-index of overall consolidation chemotherapy cycles, the total c-D-index of overall consolidation chemotherapy cycles, the total D-index from induction to consolidation chemotherapy, and the total c-D-index from induction to consolidation chemotherapy. The total c-D-index in patients with pulmonary infection was defined as the sum of the D-index of previous chemotherapy cycles and the c-D-index of a chemotherapy cycle with infection.
Figure 1. D-index in each chemotherapy cycle (A) and total neutropenia indexes (B) of a hypothetical patient. (A) D-index in each chemotherapy cycle. If the duration of neutropenia is 11 days, the expected neutrophil area (shaded area, Ae) is 11 × 500 = 5500. If the area under the neutrophil curve calculated by the trapezoidal method (striped area, Ao) is 1085, the D-index = 5500 − 1085 = 4415. (B) Total neutropenia indexes; total D-index of whole consolidation chemotherapy (CTx) cycles, total D-index from induction to consolidation CTx, and total c-D-index from induction to consolidation CTx.
Pulmonary infection was defined as new pulmonary infiltrate observed by chest X-ray or chest computed tomography (CT) regardless of microbiological evidence. In our daily clinical practice, we performed chest CT when patients had respiratory symptoms or persistent fever after empirical antibiotic therapy even without any abnormality in chest X-ray to avoid treatment delay.
Statistical considerations
We divided the patients into those with and without pulmonary infection. We assessed the impact of neutropenia indexes on pulmonary infection along with other epidemiological and clinical factors between these two groups. Dichotomous variables were compared using Fisher's exact test, and continuous variables were compared using the Student t test or Mann–Whitney U test. A P value of <0.05 was considered to be significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University (accessed 1 March 2012) at http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0). More precisely, it is a modified version of R commander (version 1.6-3) that was designed to add statistical functions that are frequently used in biostatistics.
Results
Patients
The clinical and epidemiological characteristics of the patients are shown in . Seven patients who had a history of pulmonary infection before consolidation chemotherapy were excluded from the comparative analyses. Among the remaining 26 patients without a history of pulmonary infection, five developed pulmonary infection during the first (n = 1), second (n = 2), and third (n = 2) consolidation chemotherapies with HDAC, respectively, which included one probable and four possible invasive pulmonary mold infections according to the EORTC/MSG revised criteria.Citation11 All patients were successfully treated with voriconazole or liposomal amphotericin B.
Table 1. Clinical and epidemiological characteristics of the study patients
None of the patients died during HDAC in this study. Non-hematologic toxicity was mild. Cytarabine-associated exanthema and kerato-conjunctivitis were seen in 28% and 15% of all 75 HDAC cycles, respectively. Grade III hepatotoxicity and neurologic toxicity were seen in 2 and 1 consolidation cycles, respectively.
Neutropenia indexes
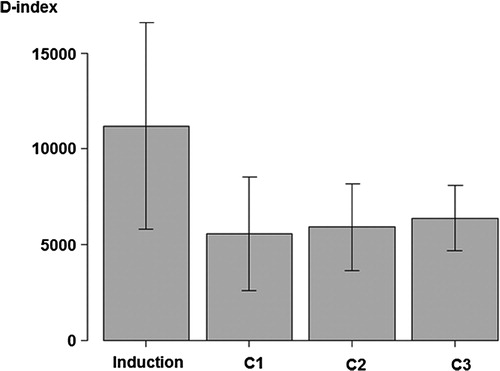
The D-index of each chemotherapy cycle of induction and consolidation chemotherapy is shown in . The D-index of induction chemotherapy was higher than that of consolidation chemotherapy (induction vs. first cycle of consolidation; 11189 ± 5405 vs. 5583 ± 3019, P < 0.001). By repeated measures analysis of variance in 18 patients who completed three courses of HDAC, the D-index tended to increase as the number of HDAC increased (4877 ± 2277, 5763 ± 2108, 6112 ± 1543, P = 0.115). The duration of neutropenia (<500/μl) showed the same tendency (12.2 ± 6.4, 13.4 ± 4.9, 15.0 ± 4.6, P = 0.117). A low neutrophil count of less than 3400/μl before consolidation chemotherapy was associated with a high D-index during consolidation chemotherapies (P = 0.040).
Figure 2. D-index of induction and consolidation chemotherapy cycles. The bar graph shows the average of the D-index of induction and consolidation chemotherapy cycles, and error bars indicate the standard deviation around the mean. C1, first cycle of consolidation chemotherapy; C2, second cycle of consolidation chemotherapy; C3, third cycle of consolidation chemotherapy.
The D-index, days of neutropenia and days of profound neutropenia in the five chemotherapy cycles among patients with pulmonary infection were 4850 ± 1096, 11.0 ± 2.3, and 8.8 ± 1.9, respectively, which were not different from those in patients without pulmonary infection. The maximum and the minimum values of the c-D-index in those five chemotherapy cycles were 5900 and 3084, respectively. Only one patient developed pulmonary infection before the c-D-index value reached 4000. The patient developed fever on the fourth day of neutropenia and then, probable invasive pulmonary aspergillosis on the seventh day of neutropenia in the third cycle of HDAC. The D-index in the second cycle of HDAC was 7143.
There was also no difference in the total D-index in the overall consolidation chemotherapy cycles between these two groups (P = 0.616). On the other hand, the total D-index from induction to consolidation chemotherapies was significantly higher in patients with pulmonary infection than in those without it (P = 0.014). However, there was no significant difference in the total c-D-index from induction to consolidation chemotherapies (P = 0.257). If we only consider induction chemotherapy, the D-index of induction chemotherapy was significantly higher in patients with pulmonary infection than in those without it (P = 0.019) ().
Table 2. Neutropenia indexes
We also evaluated the impact of the D-index of induction chemotherapy on the development of pulmonary infection during induction in this study population. Three patients who had already had pulmonary infection at the time of initial presentation were excluded from the analysis. As a result, there was no difference in the D-index of induction chemotherapy between patients with pulmonary infection during induction chemotherapy (n = 4) and those without it (n = 26) (13586 ± 12477 vs. 10846 ± 4089, P = 0.377).
As with our previous study,Citation3 we also evaluated the impact of the D-index on bloodstream infection. As a result, it could not predict bloodstream infection in the patients of this study. During the first cycle of HDAC, 9 of 33 patients developed bloodstream infection. D-index of these 9 patients was lower than that of patients without bloodstream infection (3686 ± 1407 vs. 6325 ± 3174).
Discussion
Our results showed that the D-index in each consolidation chemotherapy cycle did not predict the development of pulmonary infection. This was different from the results in AML patients undergoing induction chemotherapies as reported by Portugal et al.,Citation2 who wrote that the D-index and c-D-index were significantly higher in patients with IMI than in controls, and, for a cut-off value for the c-D-index of 5800, and a prevalence of IMI of 5, 10, and 15%, the negative predictive values were 99, 98, and 97%, respectively. A plausible explanation for this discrepancy is the difference in the time course of the neutrophil curve between induction and consolidation chemotherapies. The duration of neutropenia was shorter, as in previous reports,Citation7–Citation10 and the D-index in each cycle was lower in consolidation than in induction chemotherapies. The D-index exceeded 5800 in only one of the five chemotherapy cycles among patients with pulmonary infection in this study. Furthermore, neutropenia during previous chemotherapy cycles may have a cumulative effect. Hammond et al.Citation12 stated that the number of days of neutropenia treated as a time-dependent covariate, was independently associated with the development of invasive fungal disease in the first 100 days after induction chemotherapy for acute leukemia.
In this study, the total D-index from induction to consolidation chemotherapies was significantly higher in patients with pulmonary infection than in those without it. Furthermore, there was a significant difference in the D-index in induction chemotherapy between these two groups. These results suggest that the cumulative effect of neutropenia, especially during induction chemotherapy, might determine the risk of pulmonary infection during consolidation chemotherapies. It is possible that subclinical infection or colonization might occur during induction chemotherapy. Therefore, routine high-resolution chest CT before proceeding to consolidation, even for patients without any symptoms of pulmonary infection, may be useful for the early detection of subclinical pulmonary infection in patients with a high D-index in induction chemotherapy.
Because of the high negative predictive value of the c-D-index, it may play an important role in decreasing the unnecessary empirical antifungal therapy in these patients.Citation2,Citation3 Empirical antifungal therapy is generally recommended for high-risk hematological patients with persistent fever after 4 to 7 days of empirical antibiotic therapy,Citation13 but it induces unnecessary use of antifungal agents. Avoiding empirical antifungal therapy among patients with low D-index is a possible strategy to decrease the use of antifungal agents. Preemptive antifungal therapy based on the serial monitoring for signs of infection by radiographic studies, aspergillus galactomannan test, and so on, is another approach to decrease the unnecessary administration of antifungal agents, but a randomized controlled trial showed that the incidence of invasive fungal infection was increased among patients with profound neutropenia.Citation14 Therefore, we believe that the preemptive therapy should be combined with empirical therapy for patients with high D-index value to safely decrease the unnecessary use of antifungal agents. We are planning a randomized controlled trial to evaluate this strategy. Although the D-index and c-D-index in each chemotherapy cycle did not predict the development of pulmonary infection in this study, there is little possibility of pulmonary infection or IMI in patients with c-D-index of less than 4000 after chemotherapy for AML and HSCT, according to the results of this study and previous reports.Citation2,Citation3 Based on these data, empirical antifungal therapy will be safely withheld until the c-D-index exceeds 4000. However, median c-D-index on persistent fever on the fourth day of empirical antibiotic therapy was 4146 in the first cycle, 3755 in the second cycle, and 4426 in the third cycle, respectively, in this study population, which meant that empirical antifungal therapy with cut off value of 4000 would not lead to significant reduction of antifungal agents use. Therefore, in a clinical trial of c-D-index-guided preemptive antifungal therapy which we are planning, we set a cut off value of c-D-index as 5500 based on our previous data.Citation3 Empirical antifungal therapy will be withheld until the c-D-index value exceeds 5500, but antifungal therapy will be started preemptively by monitoring radiographic and blood tests. The important point is that we have to be careful for subclinical infection or colonization before chemotherapy. One patient in this study developed invasive pulmonary aspergillosis before c-D-index reached 4000. We suspected that there was subclinical infection before chemotherapy, because the D-index of previous cycle was as high as 7143 and the onset of invasive aspergillosis was early after the development of fever and neutropenia.
This study has some limitations. The first is the small number of patients evaluated, especially the number of patients with pulmonary infection. D-index would have a significant value to predict pulmonary infection during consolidation chemotherapy, if the study was expanded to a larger patient population. Although it might be difficult to draw definitive conclusions from these results, we believe that the results of this study suggested the necessity of careful monitoring of subclinical pulmonary infection after chemotherapy with high D-index. Second, the predictive value of neutropenia indexes might vary depending on the anti-fungal prophylaxis. Although none of the patients in the study by Portugal et al. received anti-mold prophylaxis, most of the patients in the current study received itraconazole. In this study, as opposed to Portugal's study, the D-index of induction chemotherapy did not predict the development of pulmonary infection during induction chemotherapy. This discrepancy may be due, at least partly, to the use of anti-mold prophylaxis in this study. Another possible explanation is that patients with induction failure after two courses of induction chemotherapy, who generally had a high D-index and were at high risk for infection, were excluded from this study. Finally, because the patient population of this study was limited to younger patients under 65 years old, the meaning of the D-index in the elderly patients, who are generally at higher risk for infection, could not be indicated from the results of this study.
Conclusion
The D-index in each chemotherapy cycle did not predict the development of pulmonary infection during consolidation chemotherapies with HDAC. The cumulative effect of neutropenia including induction chemotherapy might determine the risk of pulmonary infection. Routine chest CT before consolidation chemotherapy may be useful for the early detection of pulmonary infection in patients with a high D-index in induction chemotherapy. We are planning a clinical trial of c-D-index-guided preemptive antifungal therapy with a cut off value of 5500 in high-risk hematological patients, being careful for subclinical infection before chemotherapy.
References
- Safdar A, Armstrong D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: neutropenia, humoral, and splenic defects. Clin Infect Dis. 2011;53(8):798–806.
- Portugal RD, Garnica M, Nucci M. Index to predict invasive mold infection in high-risk neutropenic patients based on the area over the neutrophil curve. J Clin Oncol. 2009;27(23):3849–54.
- Kimura S, Oshima K, Sato K, Sato M, Terasako K, Nakasone H, et al. Retrospective evaluation of the area over the neutrophil curve index to predict early infection in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16(10):1355–61.
- Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331(14):896–903.
- Byrd JC, Ruppert AS, Mrozek K, Carroll AJ, Edwards CG, Arthur DC, et al. Repetitive cycles of high-dose cytarabine benefit patients with acute myeloid leukemia and inv(16)(p13q22) or t(16;16)(p13;q22): results from CALGB 8461. J Clin Oncol. 2004;22(6):1087–94.
- Pagano L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010;95(4):644–50.
- Lewis G, Hall P, Eisa N, Deremer D, Dobbins R, El-Geneidy M, et al. Acute myelogenous leukemia patients are at low risk for invasive fungal infections after high-dose cytarabine consolidations and thus do not require prophylaxis. Acta Haematol. 2010;124(4):206–13.
- Bohm A, Piribauer M, Wimazal F, Geissler K, Gisslinger H, Knobl P, et al. High dose intermittent ARA-C (HiDAC) for consolidation of patients with de novo AML: a single center experience. Leuk Res. 2005;29(6):609–15.
- Palmieri S, Sebastio L, Mele G, Annunziata M, Annunziata S, Copia C, et al. High-dose cytarabine as consolidation treatment for patients with acute myeloid leukemia with t(8;21). Leuk Res. 2002;26(6):539–43.
- Gupta A, Singh M, Singh H, Kumar L, Sharma A, Bakhshi S, et al. Infections in acute myeloid leukemia: an analysis of 382 febrile episodes. Med Oncol. 2010;27(4):1037–45.
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21.
- Hammond SP, Marty FM, Bryar JM, DeAngelo DJ, Baden LR. Invasive fungal disease in patients treated for newly diagnosed acute leukemia. Am J Hematol. 2010;85(9):695–9.
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56–93.
- Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis. 2009;48(8):1042–51.
