Abstract
Objective
To screen two important FLT3 mutations (internal tandem duplication (ITD) and D835 point mutations) in chronic myeloid leukemia (CML) patients from Southern India and report their incidence.
Methods
Screened 350 CML patients and 350 controls for the two FLT3/mutations through polymerase chain reaction and restriction fragment length polymorphism methods.
Results
ITDs were detected in 12 of the 350 CML patients (3.4%) and D835 mutations in only four cases (1.14%), relatively low in frequency as compared to those reported earlier from non-Indian populations. None of the cases showed simultaneous occurence of both ITD and D835 mutations.
Discussion
These FLT3 mutations seem to be very rare in CML, and it is possible that these could be found only in a subset of patients who are in the progressive stage and/or with varied drug response. Prospective studies are needed to confirm the role of FLT3 mutations in CML pathogenesis, which may help devising therapeutic interventions.
Introduction
Chronic myeloid leukemia (CML) is one of the hematological malignancies in Asia, accounting for 15–20% of all adult leukemias. It is a type of myeloproliferative disease characterized by the presence of Philadelphia (Ph) chromosome (with t(9; 22) (q34; q11)) with BCR-ABL fusion gene, which produces a chimeric protein with aberrant tyrosine kinase activity.Citation1,Citation2 The pathogenesis of CML involves the constitutive activation of the BCR-ABL tyrosine kinase, which governs malignant disease by activating multiple signal transduction pathways. The BCR-ABL tyrosine kinase inhibitor (TKI), imatinib, is currently the front-line treatment for CML in India although second-generation TKIs such as desatinib and nilotinib are being introduced due to emergence of drug resistance in few patients. However, the molecular mechanisms of disease progression and drug resistance remain poorly understood.
CML progression from initial chronic phase to terminal blast phase is known to involve molecular mechanisms both related and unrelated to BCR-ABL, including alterations in expression patterns of critical genes responsible for normal hematopoietic development such as FLT3. The human FLT3 gene is located on chromosome 13q12 and encompasses 24 exons spanning around 97 Kbp. FLT3 (Feline McDonough Sarcoma (Fms), like tyrosine kinase 3) is a cytokine receptor that belongs to the class III receptor tyrosine kinase family,Citation3 and is mainly expressed by immature hematopoietic cells.Citation4 Structurally, FLT3 protein is composed of five immunoglobulin-like domains in the extracellular region, a single transmembrane sequence, and a short intracellular juxtamembrane (JM) region followed by an interrupted kinase domain. It regulates a number of cellular processes such as phospholipid metabolism, transcription, cell proliferation, and apoptosis, and its activation is known to play a critical role in regulating normal hematopoiesis and cellular growth.Citation5 Aberrant activation of FLT3 kinase leads to activation of its downstream proliferative signaling pathways, including the Ras/MAPK kinase/extracellular signal-regulated kinase, PI3K/Akt, and STAT5,Citation6–Citation9 which are known to mediate leukemic cell growth.Citation10,Citation11 Further, oncogenic FLT3 signaling influences expression and function of several myeloid transcription factors indirectly blocking myeloid differentiation in the development of leukemia.Citation12 Several studies reported that FLT3 is expressed at high levels in a spectrum of hematological malignancies including CML. Internal tandem duplications (ITDs) in the JM region of the FLT3 receptor and D835 mutations in the second tyrosine kinase domain (TKD) of the same are the two distinct classes of activating mutations prevalent in leukemias.Citation13,Citation14 The pathogenecity of these mutations is quite complex and heterogeneous, being dependent upon mutation type, its expression level, size, and allelic ratio.Citation11 Most of these mutations were reported to be associated with increased disease relapse rate and reduced overall survival of the leukemia patients.Citation15–Citation20
ITDs are duplications or insertions of sequence repeats that range from 3 to 400 bp each. Generally these mutations reside in exon 14 of the gene, but sometimes it can involve intron 14 and exon 15. Although their location and length varies in individuals, they always maintain a head-to-tail orientation and preserve the reading frame. These mutations cause constitutive receptor activation of FLT3 that leads to activation of downstream signaling pathways and aberrant cell growth. More often, they were detected in leukemic patients with normal karyotype.Citation19 The second most common type of FLT3 mutations are found in exon 20 in the activation loop of the TKD, hence designated as activating loop mutations (ALMs). Majority of the ALMs were found to occur in codon 835. They induce constitutive tyrosine phosphorylation leading to activation of the receptor tyrosine kinase and are supposed to represent gain-of-function mutations. Both ITDs and ALMs lead to deregulation of FLT3 signal transduction pathways that inhibit apoptosis and promote myeloid cell proliferation.Citation20,Citation21
Although FLT3 mutations were found to be most prevalent in acute myeloid leukemia (AML; 7–23%), they are relatively less common in CML, MDS (myelodysplastic syndrome), ALL (acute lymphoblastic leukemia), and MLL (mixed lineage leukemia) (ranging between 1 and 10%). However, studies on FLT3 mutations in CML are extremely rare and none of them reported mutation in CML patients.Citation22–Citation24 Given very few earlier studies on FLT3 variants in CML, particularly none from India, it would be useful if the CML patients from different parts of India and from different ethnicities are screened for FLT3 mutations. Further, FLT3 mutations are known to induce myeloproliferative diseases in transgenic mouse model.Citation25 Keeping in view the role of FLT3 mutations in myeloproliferative diseases like AML and CML, novel therapeutic strategies are being developed for leukemia which involve several FLT3 inhibitors as drug targets.Citation26 This would be pertinent because of the several promising FLT3 inhibitors currently being developed which may be more effective as combinational therapy in imatinib-resistant CML cases. As a pioneering effort, we screened two important FLT3 mutations (ITD and D835) in large samples of CML patients and controls from Southern India and report their incidence.
Materials and methods
A total of 350 CML patients (206 males and 144 females) and 350 normal healthy controls (182 males and 168 females) were recruited. The subjects were in the age range of 12–65 years, with a median age of 42 years. The patients were enrolled from Nizams Institute of Medical Sciences, Hyderabad, during 2005–2011. All the patients were screened cytogenitically for presence of Ph chromosome as well as molecularly for the detection of BCR-ABL gene expression. Most of CML patients recruited were from the state of Andhra Pradesh and represent homogeneous ethnic and linguistic category. The controls were selected in such a way that they had no family history of any cancer, and were recruited randomly from various residential localities of the state. The study protocol was approved by the Institutional Ethical Committee as well as the Research Committee of the hospital. After obtaining informed written consent and by using prescribed proforma, essential epidemiological and clinical details were collected from each patient through personal interviews. At diagnosis, 5 ml of blood was collected in EDTA vacutainers from each of the patients and genomic DNA was extracted by rapid non enzymatic method.Citation27 The concentration of DNA was determined and then working dilutions were made for further molecular analysis.
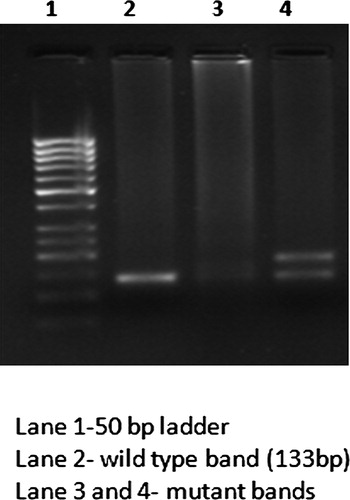
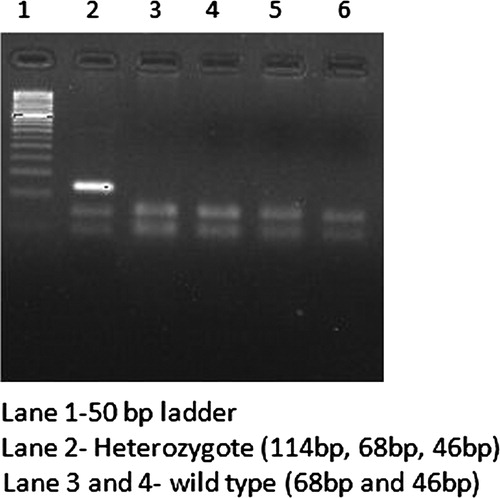
DNA samples were analyzed for FLT3/ITD and D835 mutations through polymerase chain reaction (PCR) method using specific predesigned primers as listed in .Citation23,Citation24 The amplified PCR products of ITD were checked on 3% agarose gel electrophoresis. The normal samples showed wild-type bands of 133 bp in length and samples showing longer PCR products were considered positive for FLT3/ITD mutation (). Detection of FLT3/D835 mutation was performed through PCR-restriction fragment length polymorphism analysis.Citation23 Amplified PCR products of size 114 bp were digested with 5 U of ECORV (New England Biolabs) at 37°C for 3 hours and genotyped on 3% agarose gel electrophoresis. The wild-type allele produces digested products of band size 68 and 46 bp, while the mutant form produces single band of size 114 bp (). The mutant ITD and D835 samples were further sequenced to identify the nature of mutations reported ( and ).
Table 1. Details of PCR for detection of FLT3 gene mutations
Results
The demographic and clinical characteristics of the CML patients are presented in . Majority of the cases (54.5%) were aged between 20 and 40 years, and an overwhelming proportion of the cases belonged to chronic phase (86%), followed by 8% in accelerated and 6% in blast phases. Of the 350 patients, 344 (98.2%) carried Ph chromosome (9/22 chromosome translocation), while 6 were Ph negative (1.8%). The relatively greater proportion of patients were from rural areas (62.8%), non-vegetarians (92%), non-consanguineous (84%), and with no positive family history of leukemia or any other cancer (87.4%; ).
Table 2. Incidence of FLT3-ITD and D835 mutations with respect to demographic variables
In this study, FLT3 gene mutations were identified in 4.5% of the total CML cases. ITDs were detected in 12 patients (3.4%), whereas D835 mutation was observed only in 4 out of 350 cases (1.14%). None of the controls were found to have either ITD or D835 mutation. Further, none of the cases harbored both ITD and D835 mutations. Interestingly, the ITD and D835 mutations observed were all heterozygous in nature carrying both wild type and mutant alleles. Further, the ITD mutations were observed mostly in males (11/12), a great majority was aged between 20 and 40 years (8/12) and belonged to rural areas, with no parental consanguinity (8/12), and positive family history (9/12). However, given extremely low incidence of ITD mutations, not much can be interpreted from their distribution with reference to the above variables (). With respect to clinical phase of CML, ITDs were more predominant in accelerated (2/28; 7.14%) and blast phases (4/21; 19.05%). Further, ITDs were observed only in Ph-positive CML cases and none in Ph-negative category.
FLT3-D835 mutation was observed only in four male patients, who were non-vegetarians with no parental consanguinity. Out of these four patients, two were aged between 20 and 40 years of age and in blast phase (2/21). One of these patients with D835 mutation was above 40 year. Other patient was 18 years old and showed poor cytogenetic and hematological responses and had positive family history of leukemia, implying that the younger patients carrying D835 mutation might have poor clinical outcome and drug reistance.
In general, the mean white blood count was found to be higher in patients with FLT3/ITD mutation (8.6 × 1010 ± 9.5 × 1010 WBC/l) when compared to those without these mutation (7.9 × 1010 ± 3.2 × 1010 WBC/l) or those with FLT3/D835 mutations (2.7 × 1010 ± 2.1 × 1010 WBC/l). Some studies have reported that FLT3 gene is associated with adverse response outcome in AML and APL (acute prolymphocytic leukemia) but multivariable analysis of these studies could not confirm it as an independent prognostic marker. Genotype–phenotype correlation might help in understanding the effect of FLT3 mutations in CML patients. Due to lack of systemic data on the treatment pattern and drug response in these patients, we failed to ascertain whether CML cases with ITD/D835 mutation exhibit different pattern of response in comparison to CML without mutation(s); therefore, not feasible to estimate prognostic value of these mutations from our study.
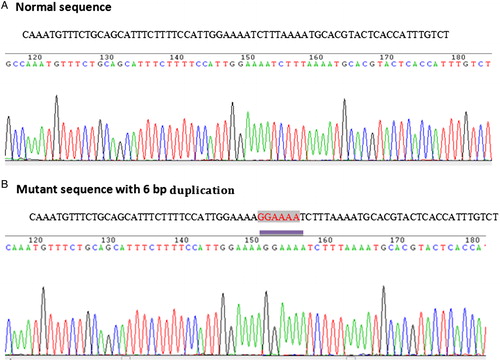
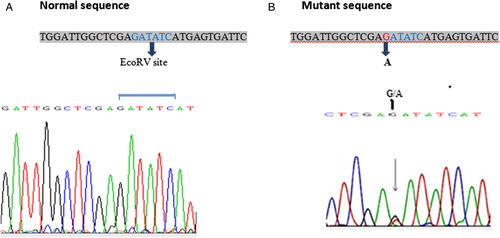
Sequence analysis of ITD and D835 mutants suggested that 2 out of 12 FLT3-ITD-positive CML patients had 6 bp duplication (), 1 had 24 bp duplication, and 4 revealed 30 bp duplication, whereas the remaining 5 cases showed duplications with additional nucleotide insertions ranging from 3 to 7 bp (). Further, all the FLT3-ITD mutations were in exon 14 of the gene and all but one were in-frame fragments with a direct head-to-tail orientation. The clinical stage of the patients with ITD mutations are given in the table (). In a study by Kayser et al., 207 (85.9%) of 241 FLT3-ITD-positive AML patients had one ITD, and 34 (14.1%) revealed more than one ITD (2 ITDs, n = 29 (12.0%); 3 ITDs, n = 3 (1.2%); 4 ITDs, n = 2 (0.8%)).Citation28 The median ITD length of above 34 multiple ITD cases was 48 nucleotides (range, 15–180 nucleotides). These duplications might disrupt the protein structure in JM domain and effect FLT3 function leading to oncogenic signaling. However, apart from ITD size, the integration site of duplications and extra insertions are important for functional characterization of these mutants and their role in CML. With regard to D835 mutation, all of them showed G → T nucleotide substitutions at ECORV site which resulted in amino acid substitution of Aspartic acid by Tyrosine at codon 835 (GAT→TAT) ().
Table 3. Characterization of FLT3 ITD mutants
Discussion
CML is a myeloproliferative disorder whose prevalence is rising at an alarming rate especially in Asian populations. It is characterized by an excessive and unregulated production of white blood cells due to a genetic abnormality involving the BCR-ABL protein. In spite of amazing success in the treatment with currently approved BCR-ABL inhibitors such as Imatinib, the management of CML still remains a scientific challenge due to acquired drug resistance and residual disease in few cases. BCR-ABL translocation is the key event that drives CML transformation but the transition of CML from the chronic phase to the terminal blast phase involves additional genetic aberrations. Therefore, identification of genes/mechanisms involved in drug resistance and progression is important to develop more effective curative drug therapies in CML. In recent years, FLT3 is identified as an important candidate gene in leukemias due to its role in the growth control of pleuripotent hematopoeitic and early progenitor cells through their signal transduction pathways. Further, increased FLT3 expression was observed in leukemia patients and leukemic cell lines. In CML, the expression of FLT3 gene was detected in 5.7% of patients in chronic phase, and in 55.9% with accelerated phase or blast crisis.Citation29 In addition, mutations in this gene are known to be associated with leukemic transformation and disease progression. Among these, ITD and D835 codon point mutations are the most common mutations reported in hematological malignances. These mutations constitutively activate FLT3 receptor kinase, independent of ligand binding, resulting in the activation of downstream prosurvival signals, and are associated with elevated blast counts, increased relapse rate, and poor overall survival.
The incidence of both FLT3/ITD and D835 mutations was found to be much lower in the Indian CML cohort when compared to most of those reported earlier among non-Indian populations, excepting a few that showed very similar incidence as that of our cohort.Citation5,Citation22–Citation24 Further, in comparison to the incidence reported so far in AML (19–23% of ITD and 3–8.8% of ALM mutations) and other hematological malignancies in India, these mutations seem to be rare in CML.Citation30–Citation32 All the mutations that we observed were in Ph-positive CML cases, none in the few Ph-negative patients. Given very low proportion of ph-negative cases in our sample, the possibility of occurrence of FLT3 mutations in such cases also cannot be totally ruled out. It may be pertinent to note that majority of the earlier studies represented only Ph-positive CML cases and did not find FLT3 mutations, hence inferred that these mutations were probably a characteristic feature of Ph-negative cases.Citation32
That the FLT3 mutations invariably occurred only in the CML patients, not in controls, implies that these variants are causative in nature, but their effect could be minor when compared to the key genetic event, BCR-ABL mutation. FLT3 mutations are capable of inducing myeloproliferative disease but may not be sufficient by itself to cause leukemia. It is likely that FLT3 mutations occur at the stem cell level and act in tandem with other mutations/variants in other candidate genes such as NPM1, WT1, and N-RAS to have role in CML progression. FLT3 mutations, particularly ITDs, not only give proliferative and antiapoptotic advantage to cells but also has a role in the differentiation arrest by influencing the expression of several critical myeloid transcription factors like C/EBPα (CAAT enhancer-binding protein alpha).Citation21
According to two hit leukemogenesis model, class 1 mutations like BCR/ABL and FLT3 gene mutations confer proliferative or survival advantage to cells through activation of the STAT, RAS/MAPK, and PI3K/AKT pathways, and are complemented by expression of proteins such as AML1/ETO that result in impaired differentiation in AML.Citation15 In this model, one class of mutations (class I) confers a proliferative or survival advantage to cells, and a second class of mutations (class II) serves primarily to interfere with hematopoietic differentiation and subsequent apoptosis of cells. It is predicted that only one mutation in each class would be required for development of leukemia but can coexist in the same patient due to additional selective advantage of mutations in cells. Both mutations can coexist in single cell or alternatively as two separate clones remaining as mutually exclusive, which may imply cooperative effects of these mutations in development of leukemia. Therefore, both the BCR/ABL and FLT3 mutations, considered as class 1 mutations can occur in the same which may also be true for FLT3 ITD and D835 mutations. These mutations can independently cause leukemia by aberrant FLT3 signaling and being mutually exclusive, they can influence leukemia development. However, the possibility of phenotypic variation due to the additive effect of these mutations is yet to be understood. Nevertheless, in view of its variable expression and role in oncogenic signaling, it is important to screen the patients routinely for FLT3 mutations and assess clinical outcome and molecular response during the CML progression.
Overall, the incidences of FLT3 mutations in CML patients seem to be relatively rare when compared to the characteristic causative BCR/ABL mutation. Although pathogenic in nature, the effect of FLT3 mutations in the origin/progression of CML appears to be minor. It is possible that these mutations could be found only in a subset of patients who are in the progressive stage during which additional genetic abnormalities accumulate. Therefore, prospective studies are needed to confirm the role of FLT3 mutations in CML, not only in advanced blast phase, but also on Ph-negative patients, and imatinib-resistant patients which can help in ascertaining the usefulness of this marker for prognosis and early therapeutic intervention. Further, exploring the mechanisms by which these FLT3 alterations lead to deregulated proliferation can provide a better understanding of the molecular pathogenesis of CML.
Acknowledgements
This study was conducted during the tenure of AS as a visiting scientist at the Biological Anthropology Unit, Indian Statistical Institute, Hyderabad. We are thankful to the Director, ISI, for logistic support. We thank Dr K. Thangaraj, CCMB, for his help in characterizing the mutant samples through direct sequencing and Shilpi Dasgupta for reading through the manuscript and for giving feedback on the presentation of the manuscript.
References
- Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497–9.
- Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–3.
- Mathews W, Jordan CT, Wiegand GW, Pardoll D, Lemischka IR. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell enriched populations. Cell. 1991;65:1143–52.
- Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–65.
- Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–9
- Spiekermann K, Bagrintseva K, Schoch C, Haferlach T, Hiddemann W, Schnittger S. A new and recurrent activating length mutation in exon 20 of the FLT3 gene in acute myeloid leukemia. Blood. 2002;100:3423–5.
- Choudhary C, Schwable J, Brandts C, Brandts C, Tickenbrock L, Sargin B, et al. AML-associated Flt3 kinase domain mutations show signal transduction differences compared with Flt3 ITD mutations. Blood. 2005;106:265–73.
- Grundler R, Miething C, Thiede C, Peschel C, Duyster J. FLT3-ITD and tyrosine kinase domain mutants induce 2 distinct phenotypes in a murine bone marrow transplantation model. Blood. 2005;105:4792–9.
- Gabbianelli M, Pelosi E, Montesoro E, Valtieri M, Luchetti L, Samoggia P, et al. Multi-level effects of flt3 ligand on human hematopoiesis: expansion of putative stem cells and proliferation of granulomonocytic progenitors/monocytic precursors. Blood. 1995;86:1661–70.
- Vempati S, Reindl C, Kaza SK, Kern R, Malamoussi T, Dugas M, et al. Arginine 595 is duplicated in patients with acute leukemias carrying internal tandem duplications of FLT3 and modulates its transforming potential. Blood. 2007;110:686–94.
- Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2007;111:2776–84.
- Zheng R, Small D. Mutant FLT3 signaling contributes to a block in myeloid differentiation. Leuk Lymphoma. 2005;46(12):1679–87.
- Nakao M, Janssen JW, Erz D, Seriu T, Bartram CR. Tandem duplication of the FLT3 gene in acute lymphoblastic leukemia: a marker for the monitoring of minimal residual disease. Leukemia. 2000;14:522–4.
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–52.
- Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–42.
- Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9.
- Schnittger S, Schoch C, Dugas M, Dugas M, Kern W, Staib P, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66.
- Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35.
- Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–80.
- Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94.
- Zheng R, Levis M, Piloto O, Brown P, Baldwin BR, Gorin NC, et al. FLT3 ligand causes autocrine signaling in acute myeloid leukemia cells. Blood. 2004;103:267–74.
- Yokota S, Kiyoi H, Nakao M, Iwai T, Misawa S, Okuda T, et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies: a study on a large series of patients and cell lines. Leukemia. 1997;11:1605–9.
- Lin P, Jones D, Medeiros LJ, Chen W, Vega-Vazquez F, Luthra R. Activating FLT3 mutations are detectable in chronic and blast phase of chronic myeloproliferative disorders other than chronic myeloid leukemia. Am J Clin Pathol. 2006;126:530–3.
- Xu F, Taki T, Yang HW, Hanada R, Hongo T, Ohnishi H, et al. Tandem duplication of the FLT3 gene is found in acute lymphoblastic leukaemia as well as acute myeloid leukaemia but not in myelodysplastic syndrome or juvenile chronic myelogenous leukaemia in children. Br J Haematol. 1999;105:155–62.
- Lee BH, Williams IR, Anastasiadou E, Boulton CL, Joseph SW, Amaral SM, et al. FLT3 internal tandem duplication mutations induce myeloproliferative or lymphoid disease in a transgenic mouse model. Oncogene. 2005;24:7882–92.
- Leung AYH, Man CH, Kwong YL. FLT3 inhibition: a moving and evolving target in acute myeloid leukaemia. Leukemia. 2013;27:260–8.
- Lahiri DK, Nurnberger JI Jr. A rapid non-enzymatic method for the preparation of HMW DNA from blood RFLP studies. Nucleic Acid Res. 1991;19:5444.
- Kayser S, Schlenk RF, Londono MC, Breitenbuecher F, Wittke K, Du J, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2380–92.
- Xu B, Tian H, Zhou SY. Detection of FLT3 gene and FLT3/ITD gene mutation in chronic myeloid leukemia and its significance. Chin J Cancer. 2004;23:1218–21.
- Nageswararao D, Senthil R, Raghunadharao D, Sugunakar V, Sailaja K, Surekha D, et al. Fms like tyrosine kinase (FLT3) and nucleophosmin 1 (NPM1) mutations in de novo normal karyotype acute myeloid leukemia (AML). Asian Pacific J Cancer Prev. 2010;11:1811–6.
- Chauhan PS, Bhushan B, Mishra AK, Singh LC, Saluja S, Verma S, et al. Mutation of FLT3 gene in acute myeloid leukemia with normal cytogenetics and its association with clinical and immunophenotypic features. Med Oncol. 2011;28:544–51.
- Ahmad F, Mandava S, Das BR. Analysis of FLT3-ITD and FLT3-Asp835 mutations in de novo acute myeloid leukemia: evaluation of incidence, distribution pattern, correlation with cytogenetics and characterization of internal tandem duplication from Indian population. Cancer Invest. 2010;28:63–73.