Abstract
Objective
Hepcidin is a key regulator of body iron homeostasis. The inflammatory cytokine interleukin (IL)-6 has been reported to upregulate expression of the hepcidin (HAMP) gene in monocytes. The purpose of this work was to determine HAMP expression at steady state in monocytes of splenectomized and non-splenectomized patients with HbE-β-thalassemia compared with normal controls.
Methods
Levels of HAMP mRNA were measured using real-time reverse transcriptase polymerase chain reaction. Plasma IL-6, soluble transferrin receptor (sTfR), and ferritin levels were determined by enzyme-linked immunosorbent assay, and C-reactive protein (CRP) by nephelometry.
Results
Levels of HAMP mRNA, CRP, IL-6, sTfR, and ferritin were significantly higher in both groups of patients with thalassemia than controls, but were not different between splenectomized and non-splenectomized patients. Monocyte HAMP mRNA content of patients with thalassemia correlated with plasma IL-6 and CRP levels.
Discussion
Patients with HbE-β-thalassemia have persistent elevation of the plasma inflammatory cytokines, CRP, and IL-6, and the latter could be responsible (in part) to the induction of HAMP expression in monocytes of patients with HbE-β-thalassemia.
Introduction
Hepcidin, a key regulator of body iron homeostasis is synthesized mainly from hepatocytes in the liver, after which it enters the blood circulation and is finally excreted into the urine.Citation1–Citation3 However, it is also produced from various organs and tissues, including kidney, adipose tissue, macrophages, and monocytes, but at a lower level than by the liver,Citation4–Citation7 and the role of hepcidin in cell types other than hepatocytes is unclear. Hepcidin acts by binding to plasma membrane iron exporter, ferroportin, causing internalization and degradation of this iron transporter, and resulting in reduction of iron release from macrophages and hepatocytes, thereby decreasing the level of iron in the plasma.Citation8
Hepcidin is also known to be an antimicrobial peptide. Transcription of hepcidin (HAMP) gene is upregulated by infection and inflammation.Citation9 In hepatocyte cultures, expression of the HAMP gene is induced by interleukin (IL)-6.Citation10 Downregulation of HAMP mRNA expression is caused by anemia and hypoxia.Citation11
Thalassemia is an autosomal recessive anemia resulting from a decrease in or absence of synthesis of the α-globin chain (α-thalassemia) or the β-globin chain (β-thalassemia).Citation12 The chronic anemia, together with premature removal of the affected red blood cells, results in iron overload, which leads to increased susceptibility to opportunistic bacterial infection and impaired immunity.Citation13,Citation14 Splenectomized patients with thalassemia are at higher risk of infection than non-splenectomized patients with thalassemia,Citation15 as indicated by higher levels of inflammatory markers; C-reactive protein (CRP),Citation16 and IL-6.Citation17 However, recent prospective controlled study in children with HbE-β-thalassemia has questioned this notion. The infection rates were not significantly different between control subjects and patients with either severe (including splenectomized patients) or non-severe HbE-β-thalassemia who received regular blood transfusions and iron chelation.Citation18 In addition, the results from the study of Origa et al.,Citation19 who examined the degree of iron deposition in liver biopsy samples and hepcidin levels in the urine of patients with thalassemia major who received regular transfusion therapy and chelation, showed that hepcidin levels were increased and iron was deposited mainly in Kupffer cells (liver macrophages), whereas few hemosiderin granules were found in hepatocytes. Another study of patients with β-thalassemia major receiving regular transfusions found that hepatic HAMP mRNA levels correlated with urinary hepcidin levels but did not correlated with iron concentration in the liver.Citation20 Thus, we aimed to investigate the expression of HAMP mRNA in monocytes of splenectomized and non-splenectomized patients with HbE-β-thalassemia in relation to their levels of inflammatory makers.
Methods
Ethics approval
The research was approved by Khon Kaen University Ethics Committee for Human Research (HE531289), and informed consent was obtained from all subjects.
Subjects
Patients with HbE-β-thalassemia (30 splenectomized and 29 non-splenectomized) who attended Srinagarind Hospital, Khon Kaen University, Thailand for regular blood transfusions (3 to 4 weeks intervals) and 27 normal subjects were recruited for this study. None of the patients had any signs of infection and/or inflammation upon physical examination on the day of blood collection. The steady-state hemoglobin (Hb) level was taken as the average of pre-transfusion Hb levels from blood samples collected at three consecutive appointments for transfusion. The criteria for normal controls were Hb levels ≥13 g/dl for males and ≥12 g/dl for females; mean corposcular volume (MCV) >80 fl; negative results for osmotic fragility and dichlorophenol-indophenol (DCIP) tests;Citation21 Hb typing A2A, %A2 <4%; and serum ferritin ≥10 µg/l. The biological data of patients and control subjects are shown in .
Table 1. Characteristics of HbE-β-thalassemia patients and normal controls
Blood samples
Venous blood samples (10 ml) were collected into tube containing ethylenediaminetetraacetic acid. For patients with thalassemia, blood was drawn before they received the blood transfusion. Plasma samples were separated by centrifugation at 2,000 g for 10 minutes and stored at −70°C until assayed.
Assays
Hematological parameters including Hb level and type, hematocrit, MCV, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, white blood cell (WBC), and red blood cell counts were analyzed using an automated hematology analyzer (KX-21; Sysmex Corp., Kobe Japan). Ferritin, soluble transferrin receptor (sTfR) and IL-6 levels were determined by enzyme-linked immunosorbent assay (Fortress Diagnostics, Antrim, UK; BioVendor Research and Diagnostic Products, Karasek, Czech Republic; BioLegend, San Diego, CA, USA, respectively). CRP level was measured by a commercial kit based on the nephelometry principle (BN ProSpec® System; Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA).
Preparation of mononuclear cells and monocytes
Peripheral blood mononuclear cells were isolated from the fresh whole blood sample (Ficoll-Paque Premium, density 1.073; GE Healthcare Bio-Science AB, Uppsala, Sweden). Monocytes were then prepared by suspending mononuclear cells in RPMI 1640 medium (Invitrogen Corp., Carlsbad, CA, USA) supplemented with 2 nmol/l L-glutamine, 10% (v/v) heat-inactivated fetal bovine serum (JR Scientific Inc, Woodland, CA, USA), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Invitrogen Corp.), seeding the cells into 60 × 15 mm plastic dish (Corning Inc., Corning, NY, USA) and incubating in an atmosphere of 5% CO2 at 37°C for 45 minutes. Non-adherent cells were removed then the adhering monocytes were washed with phosphate-buffered saline before being harvested.Citation7
Determination of HAMP mRNA expression
Total RNA was extracted from monocytes (Omega Bio-tek, Inc., Norcross, GA, USA) and used for measurement of HAMP and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression levels by quantitative real-time reverse transcriptase polymerase chain reaction. In brief, 0.5 µg of RNA was used with a commercial kit (SensiMixTM SYBR No-ROX One-Step kit; Bioline USA Inc., Taunton, MA, USA) containing primers for human hepcidin (GCACTGAGCTCCCAGATCTG (sense) and CTACGTCTTGCAGCACATCC (antisense)) and run in a thermocycler (Rotor Gene 3000; Corbett Research, Sydney, Australia) using the following conditions: 10 minutes at 42°C; 10 minutes at 95°C; and 50 cycles at 95°C for 15 seconds, 58°C for 15 seconds, and 72°C for 20 seconds. The primer sequences for GAPDH were GAAGGTGAAGGTCGGAGTC (sense) and GAAGATGGTGATGGGATTTC (antisense). The Ct value (the exponential term determined from a log-linear plot of the real-time quantitative polymerase chain reaction signal versus the cycle number) was recorded. The mean Ct values of both HAMP and GAPDH from tripicate experiments were used to calculate the relative HAMP expression in each sample and the fold change in each patient's HAMP expression, normalized to GAPDH and relative to the expression in normal controls was calculated by the 2−ΔΔCt method.Citation6,Citation22
Statistical analysis
Data are reported as mean ± standard deviation (SD). Statistical analysis was performed using the SPSS statistical package (version 17; SPSS Inc., Chicago, IL, USA). Comparisons between splenectomized, non-splenectomized patients with thalassemia and the normal controls were carried out using the Kruskal–Wallis test and between splenectomized and non-splenectomized patients or patients in each group with normal controls by Mann–Whitney U-test. Correlations were calculated using the Spearman rank test, and P < 0.05 was considered statistically significant.
Results
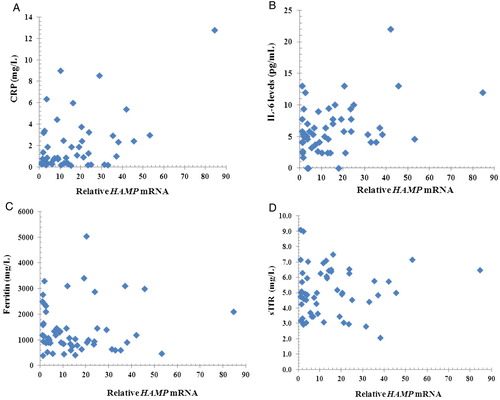
The characteristics of patients with HbE-β-thalassemia and normal subjects are showed in . There were no significant differences between the two patient groups in either steady-state Hb concentration or volume of blood received. The WBC counts of both splenectomized and non-splenctomized patients with thalassemia were within the normal range (mean ± 2SD or 95% range = 9.0 ± 4.0 × 109/l).Citation23 Compared with normal subjects, mean concentrations of the inflammatory markers, CRP and IL-6, were significantly higher in both splenectomized and non-splenectomized patients (CRP: P = 0.001 and P = 0.024, respectively; IL-6: P < 0.001 for both patient groups). Similarly, plasma ferritin levels and sTfR levels in splenectomized and non-splenectomized patients were significantly increased (P < 0.001 for both patient groups) compared with normal controls. However, there were no significant differences in levels of CRP, IL-6, ferritin, or sTfR between splenectomized and non-splenectomized patients with thalassemia (P = 0.165, 0.663, 0.176, and 0.275, respectively). Mean HAMP mRNA levels in monocytes of splenectomized and non-splenectomized patients with thalassemia were 12.5 and 19.8 times, respectively, above those of normal controls. There was no difference in HAMP mRNA levels between the two groups of patients with thalassemia (P = 0.199). It is noteworthy that in the patients with thalassemia, monocyte HAMP mRNA levels were found to be correlated with plasma CRP (R = 0.625, P < 0.001) and IL-6 (R = 0.272, P = 0.047), but not with plasma ferritin (R = 0.008, P = 0.954) and sTfR (R = −0.015, P = 0.277) ().
Figure 1. Scatter plots between relative HAMP mRNA and CRP, IL-6, ferritin, sTfR. Scatter plots between relative HAMP mRNA and CRP (A), IL-6 (B), ferritin (C), and sTfR (D). Relative HAMP mRNA levels (normalized to GAPDH relative to normal controls) correlated with CRP (R = 0.625, P < 0.001), IL-6 (R = 0.272, P = 0.047) but did not correlated with ferritin (R = 0.008, P = 0.954) and sTfR (R = −0.015, P = 0.277).
Discussion
In this study, we found that the chronic anemia in splenectomized and non-splenectomized patients with thalassemia was corrected by regular blood transfusions at intervals of 3–4 weeks. Splenectomized and non-splenectomized patients who had pre-transfusion Hb of less than 9 g/dl were given 10–15 ml/kg of leucocyte-poor red blood cells, in accordance with the recommendations for transfusion programs by the Thalassemia International FederationCitation24 to maintain the pre-transfusion Hb level above 9–10.5 g/dl. Therefore, the Hb levels of splenectomized patients and the volume of blood they received were not significantly different from those of non-splenectomized patients (). Although most patients with thalassemia received iron-chelation therapy (20–40 mg/kg, desferrioxamine (Desferal®; Novartis Pharmaceuticals Corp. East Hanover, NJ, USA) subcutaneous infusion over a period of 8–12 hours, 3–7 days/week; and/or 50–75 mg/kg/day deferiprone (GPOL1®; local made deferiprone by the Government Pharmaceutical Organization of Thailand) orally in two to three divided doses daily when ferritin levels rose above 1000 µg/l24), plasma ferritin levels in both groups of patients remain significantly higher than normal subjects.
Iron store and inflammatory stimuli activate HAMP mRNA expressionCitation8,Citation10,Citation11,Citation25 whereas erythropoietic activity downregulated it.Citation20 Soluble transferrin receptor, a truncated form of transferrin receptor found in the blood in proportional to the amount found on the surface of erythroid precusors, is used as an index of erythropoietic activity.Citation26 It has been shown to be age-dependent; however, the upper limit of the reference values in healthy children at different age groups from 1 to 16 years was less than 3.3 g/l.Citation27 In our study, sTfR levels in splenectomized and non-splenectomized patients were higher than the reference value, and there was no significant difference between the two patient groups, even though the patients in the non-splenectomized group were significantly younger. The steady-state Hb levels of the two groups were also not significantly different. This may be due to the correction of ineffective erythropoiesis by regular transfusions. Levels of sTfR in patients with HbE-β-thalassemia in this study were not correlated with HAMP mRNA levels in monocytes. Similarly, although plasma ferritin levels of both splenectomized and non-splenectomized patients were much higher than those of normal controls, there was no statistical difference between the two patient groups. There was also no correlation between ferritin levels and HAMP mRNA expression in the monocytes of patients with thalassemia who received regular transfusions. These results suggest that erythropoietic drive and body iron store may not play a key role in upregulation of HAMP expression in monocytes of patients with HbE-β-thalassemia who receive regular transfusions.
In addition to its role as a regulator of iron homeostasis, hepcidin has antibacterial activity properties.Citation2 Upon infection, lipopolysaccharides induce macrophages to release IL-6 in order to eliminate bacteria and this cytokine in turn stimulates the liver to produce CRP.Citation28 CRP is a good indicator of inflammation or infection. In this study, plasma IL-6 levels and CRP levels were significantly higher in patients with thalassemia than in normal subjects, but were lower than the levels found in patients with acute inflammation or infection (CRP >40 mg/l).Citation29 Furthermore, chronic inflammation from endothelial cell activation and hemostatic alterations, which are ongoing processes in thalassemia, lead to an increase in IL-6 and CRP production.Citation30,Citation31 Our results are in agreement with those of previous reports.Citation16,Citation17 However, we found that CRP and IL-6 levels were not significantly different between splenectomized and non-splenectomized patients, whereas a previous study showed that splenectomized patients had higher CRP levels than non-splenectomized patients.Citation16 It is worth noting that this latter report contained no details regarding blood transfusion or iron chelation treatment. One explanation for this difference could be that patients in our study may have benefited from the treatment with regular transfusions and appropriate iron chelation. A recent prospective study on patients with HbE-β-thalassemia, including patients recruited for this study, reported no significant difference in infection rate compared with normal controls, suggesting that regular blood transfusions and appropriate iron chelation therapy can decrease infections in pediatric patients with HbE-β-thalassemia.Citation18
Production of hepcidin from the liver most likely accounts for the level of hepcidin found in the blood.Citation9 The expression of HAMP by monocytes suggests local interaction between hepcidin and ferroportin in an autocrine fashion. The increased expression of HAMP mRNA in the monocytes of patients with HbE-β-thalassemia compared with normal individuals may reflect the existence of a covert inflammatory process in the patients, as has been found in monocytes of patients with anemia of chronic disease.Citation7 Furthermore, in our study, HAMP mRNA expression levels in patients with thalassemia correlated with levels of Il-6 and CRP. This may be due to induction of HAMP gene expression by various inflammatory stimuli, including IL-6.Citation32 A study of lipopolysaccharide-activated human monocyte THP-1 cells transiently transfected with plasmid expressing ferroportin/EmGFP fusion protein and a ferroportin mutation construct reported reduced presence of ferroportin on THP-1 cell surface, which correlated with the increase in hepcidin.Citation7 Therefore, the increased hepcidin content in monocytes of patients with HbE-β-thalassemia might lead to iron retention within cells which could contribute to reduction in iron availability for pathogen growth.
In summary, we found that in patients with HbE-β-thalassemia at steady state, there was a persistent elevation of plasma inflammatory cytokines (CRP and IL-6), which could be responsible for the increased HAMP mRNA content in monocytes. This in turn might act as an autocrine mechanism, leading to iron accumulation within cells and thereby result in reduction of iron available for pathogen growth. These results indicate an additional benefit from treating severe patients with thalassemia with regular blood transfusions and iron chelation treatment.
Acknowledgment
This work was supported by Khon Kaen University.
References
- Ganz T. Hepcidin-a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18:171–82.
- Krause A, Neitz S, Magert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–50.
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–10.
- Kulaksiz H, Theilig F, Bachmann S, Gehrke SG, Rost D, Janetzko A, et al. The iron-regulatory peptide hormone hepcidin: expression and cellular localization in the mammalian kidney. J Endocrinol. 2005;184:361–70.
- Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 2006;131:788–96.
- Sow FB, Florence WC, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. Expression and localization of hepcidin in macrophages: a role in host defense against tuberculosis. J Leukoc Biol. 2007;82:934–45.
- Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood 2008;111:2392–9.
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090–3.
- Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–42.
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 2003;101:2461–3.
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44.
- Weatherall DJ, Clegg JB. The thalassemia syndromes. 4th ed. Oxford: Blackwell; 2001.
- Walker EM Jr., Walker SM. Effects of iron overload on the immune system. Ann Clin Lab Sci. 2000;30:354–65.
- Wanachiwanawin W. Infections in E-beta thalassemia. J Pediatr Hematol Oncol. 2000;22:581–7.
- Aswapokee N, Aswapokee P, Fucharoen S, Wasi P. A study of infective episodes in patients with beta-thalassemia/Hb E disease in Thailand. Birth Defects Orig Artic Ser. 1987;23:513–20.
- Archararit N, Chuncharunee S, Pornvoranunt A, Atamasirikul K, Rachakom B, Atichartakarn V. Serum C-reactive protein level in postsplenectomized thalassemic patients. J Med Assoc Thai. 2000;83 (Suppl. 1):S63–9.
- Chuncharunee S, Archararit N, Hathirat P, Udomsubpayakul U, Atichartakarn V. Levels of serum interleukin-6 and tumor necrosis factor in postsplenectomized thalassemic patients. J Med Assoc Thai. 1997;80 (Suppl. 1):S86–91.
- Jetsrisuparb A, Jetsrisuparb C. Infections and illnesses in children with Hb E beta-thalassemia: a prospective controlled study. J Med Assoc Thai. 2010;93:48–55.
- Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92(5):583–8.
- Kattamis A, Papassotiriou I, Palaiologou D, Apostolakou F, Galani A, Ladis V, et al. The effects of erythropoetic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica. 2006;91(6):809–12.
- Fucharoen G, Sanchaisuriya K, Sae-ung N, Dangwibul S, Fucharoen S. A simplified screening strategy for thalassaemia and haemoglobin E in rural communities in south-east Asia. Bull World Health Organ. 2004;82(5):364–72.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25:402–8.
- Bain BJ, Bates I, Laffan MA, Lewis SM. Dacie and Lewis practical haematology. 11th ed. China: Churchill Livingstone; 2012.
- Cappellini M-D, Cohen A, Eleftheriou A, Piga A, Porter J, Taher A. Guidelines for the clinical management of thalassaemia. 2nd ed. Nicosia, Cyprus: TIF; 2007.
- Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–9.
- Huebers HA, Beguin Y, Pootrakul P, Einspahr D, Finch CA. Intact transferrin receptors in human plasma and their relation to erythropoiesis. Blood 1990;75(1):102–7.
- Suominen P, Virtanen A, Lehtonen-Veromaa M, Heinonen OJ, Salmi TT, Alanen M, et al. Regression-based reference limits for serum transferrin receptor in children 6 months to 16 years of age. Clin Chem. 2001;47(5):935–7.
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36.
- Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999;17(6):1019–25.
- Aggeli C, Antoniades C, Cosma C, Chrysohoou C, Tousoulis D, Ladis V, et al. Endothelial dysfunction and inflammatory process in transfusion-dependent patients with beta-thalassemia major. Int J Cardiol. 2005;105(1):80–4.
- Angchaisuksiri P, Atichartakarn V, Aryurachai K, Archararit N, Chuncharunee S, Tiraganjana A, et al. Hemostatic and thrombotic markers in patients with hemoglobin E/beta-thalassemia disease. Am J Hematol. 2007;82(11):1001–4.
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6.
