Abstract
Background
Sickle haemoglobin (HbS) is known to offer considerable protection against falciparum malaria. However, the mechanism of protection is not yet completely understood. In this study, we investigate how the presence of the sickle cell trait affects the haematological profile of AS persons with malaria, in comparison with similarly infected persons with HbAA. This study is based on the hypothesis that the sickle cell trait plays a protective role against malaria.
Methods
Children from an endemic malaria transmission area in Yemen were enrolled in this study. Hematological parameters were estimated using manual methods, the percentage of parasite density on stained thin smear was calculated, haemoglobin genotypes were determined on paper electrophoresis, ferritin was measured using enzyme-linked immunosorbent assay, serum iron and TIBC were assayed using spectrophotometer, transferrin saturation index was calculated by dividing serum iron by TIBC and expressing the result as a percentage. Haematological parameters were compared in HbAA- and HbAS-infected children.
Results
Falciparum malaria parasitaemia was confirmed in the blood smears of 62 children, 44 (55.7%) of AA and 18 (37.5%) AS, so there was higher prevalence in HbAA children (P = 0.047). Parasite density was lower in HbAS- than HbAA-infected children (P = 0.003). Anaemia was prominent in malaria-infected children, with high proportions of moderate and severe forms in HbAA (P = 0.001). The mean levels of haemoglobin, packed cell volume, reticulocyte count, platelets count, lymphocytes, eosinophils, and serum iron were significantly lower while total leukocytes, immature granulocytes, monocytes, erythrocyte sedimentation rate, transferrin saturation, and serum ferritin were significantly higher in HbAA-infected children than HbAS-infected children.
Conclusion
Infection with Plasmodium falciparum malaria caused more significant haematological alterations of HbAA children than HbAS. This study supports the observation that sickle cell trait seems to be a beneficial genetic factor that resists malaria, since inheriting it protects against significant haematological consequences of malaria.
Introduction
Malaria remains one of the most prevalent infectious diseases in the world, resulting in 225 million cases each year.Citation1 At present, the most important problem posed by malaria is the resistance of Plasmodium falciparum infection to antimalarial drugs, leading to hyperparasitaemia and development of malarial complications. Haematologic changes associated with P. falciparum malaria infection are well recognized, but specific changes may vary with malaria endemicity, background haemoglobinopathy, nutritional status, demographic factors, and malaria immunity.Citation2 The sickle β-globin gene has a geographical distribution that is virtually identical to that of falciparum malaria. Much epidemiological and cellular evidence have accrued over the years to support the hypothesis that HbS, particularly heterozygous genotype, HbAS is protective against malaria.Citation3,Citation4 The mechanisms for the protective effects have also been reviewed and probably reflect both impaired entry into, and growth of parasites in red cells.Citation5 Supportive data have focused largely on parasite rates, densities and patient mortality, and various studies showed that sickle cell trait (HbAS) provides significant protection against all causes of mortality, especially severe malarial anaemia and high-density parasitaemia.Citation3,Citation6
Worldwide, some studies have been performed to determine the influence of the HbAS genotype on different haematologic responses to malaria but no such study has been carried out in Yemen, where falciparum malaria is endemic, and sickle haemoglobinopathy is prevalent. This study was therefore to examine the effect of HbAS genotype on development of various haematological abnormalities, in comparison with HbAA Yemeni children infected with falciparum malaria.
Materials and methods
Study location and subjects
The study was conducted in TurAlbaha district hospital, Lahj governorate, south-west of Yemen. Malaria is endemic in the area with perennial transmission that peaks during and after the rainy season, from May through October. During one year (1 November 2008 to 31 October 2009), 127 children (<14 years) from paediatric out-patient attendance, who presented with clinical illness compatible with uncomplicated malaria (i.e. fever, chills, sweating, vomiting, diarrhoea, fatigue, abdominal pain, cough, nausea, anorexia and lack of nervous system involvement, and severe respiratory distress and circulatory collapse) were enrolled in the study. Informed consent was sought and obtained from parents or guardians of the children after providing them a detailed explanation of the objectives, importance, and benefits of the study. This study was approved by Lahij Governorate Public Health Office, Yemen.
Blood sampling and laboratory assessment
A standard venepuncture technique was used to collect 6 ml of blood and divided into a dipotassium EDTA bottles for haematolological investigations, and into plain tubes for serum iron markers measurement.
Malaria parasites screening and density
Double thick blood smears were prepared immediately after sampling and stained with Giemsa stain, for observation under an oil-immersion high-power field light microscope. One slide from each child was examined independently by two experienced technicians and graded as positive (asexual malaria parasites seen) or negative (no malaria parasites seen) based on the inspection of 100 fields of the thick smear. Parasites enumeration (asexual form of malaria trophozoites) was performed on thin film stained with Leishman's stain and parasitaemia density expressed as percentage in 2000 counted red blood cells. Results of the two primary readers were averaged if concurrent and used for the calculation of parasite density. Non-concurrence in species or parasite counts between the primary readers was referred to a third expert microscopist whose determination of species and parasite count was considered final.
Haematological parameters
All haematological parameters were measured, based on the methods in the Manual of Basic Techniques for a Health Laboratory, WHO.Citation7 Haemoglobin concentration was determined by the cyanmethaemoglobin spectrophotometric method, with calibration of the spectrophotometer using haemglobincyanide reference solution. Packed cell volume (PCV) was determined by the microhaemotocrit method. Anaemia was defined according to the World Health Organization's criteria of a haemoglobin level <11 g/dl in children,Citation8 and it was further classified into categories of mild (haemoglobin level 10–10.9 g/dl), moderate (haemoglobin level 7–9.9 g/dl), and severe (haemoglobin level <7 g/dl).
Leukocytes and platelets were counted under the microscope using the improved Neubaeur counting chamber. The results were expressed as leukocytes, platelets ×103/mm3. Differential leukocyte counts were the relative proportions (%) of the various types of mature and immature leukocytes. Erythrocyte sedimentation rate (ESR) was estimated within 1 hour by using a modified Westergren's tube (open at both side). Reticulocyte counts were manually done as percentages calculated, based on the counts of the reticulocytes in at least 500 red cells, counted systematically after supravital staining with brilliant cresyl blue, in a light microscopic examination.
Haemoglobin electrophoresis
The presence of Hb variants was detected on a haemolysate prepared from EDTA sample on cellulose acetate paper at pH 8.6, using a Hb electrophoresis kit (Helena Laboratories, Beaumont, TX, USA).
Iron markers assay
Serum ferritin was measured using enzyme-linked immunosorbent assay kit (INTERMEDICAL, Europa, Villaricca, Italia). Serum iron and TIBC were assayed using Spinreact spectrophotometric test kits (Spinreact, Santa Coloma, Spain), according to the manufacturer's instructions. Transferrin saturation index was calculated by dividing serum iron by TIBC and expressing the result as a percentage.
Statistical analysis
Data were analyzed using SPSS (version 18.0). The mean values and standard deviations were calculated. Inter-group haematological mean values of parameters were compared, using Students’ t-test. Pearson's χ2 analysis was used for comparing proportions. P values <0.05 were considered as statistically significant.
Results
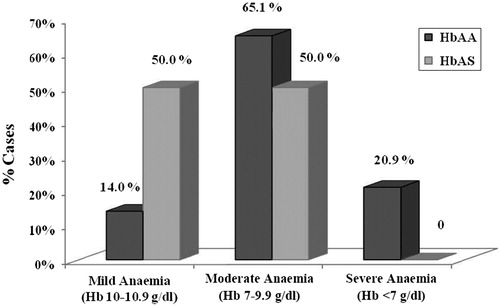
One hundred and twenty seven eligible children were enrolled in the study, of them 79 (40 males and 39 females) had HbAA genotype, and 48 (25 males and 23 females) were HbAS. Malaria (asexual form of P. falciparum trophozoite) was confirmed microscopically in 44 HbAA and 18 HbAS, with prevalence of 55.7 and 37.5%, respectively (P = 0.047). The remaining children (35 HbAA and 30 HbAS) were negative and were used as controls. Male/female ratio and mean ages of the parasitaemics and nonparasitaemic children (controls) were similar (). Parasite density was significantly lower in HbAS than HbAA (1.7 ± 0.8 vs. 5.2 ± 4.4 parasites%; P = 0.003). Haematological parameters were compared in parasitaemic and non-parasitaemic children (controls) of same genotype, and in parasitaemic children of different genotypes (HbAA vs. HbAS) (). Anaemia was seen in 57 (91.9%) of parasitaemic children (75.4% HbAA and 24.6% HbAS, P = 0.001). The prevalence of parasitaemic children by genotype was cross-tabulated with severity of anaemia (). A greater proportion of HbAA children had moderate and severe anemia (P = 0.009). The mean values of haemoglobin concentration, PCV, reticulocyte count, platelets count, lymphocytes, and eosinophils were lower, while the means of total leukocytes, immature granulocytes (bands, metamyelocytes), monocytes, and ESR were higher in parasitaemic children than non-parasitaemic, with significant differences in HbAA. Iron parameters studied showed significantly lower serum iron and TIBC in parasitaemic children than non-parasitaemic. Transferrin saturation and serum ferritin were significantly higher in HbAA-infected children and lower in HbAS ().
Table 1. Haematological parameters in parasitaemic and non-parasitaemic children of HbAA and HbAS genotypes
Discussion
The haematological parameters alterations are common in malaria, and reported to be most pronounced in P. falciparum infection, but the extent of alterations differ, depending on genetic, epigenetic, and environmental factors. Positive malaria smear was seen in 52% of studied children. The prevalence of malaria was lower in HbAS, compared with HbAA children and associated with significantly reduced parasitaemia level (P = 0.003). Similarly, a number of studies have shown either a lower prevalence or a lower density of parasitaemia among children with HbAS, compared with HbAACitation9,Citation10 Less susceptibility of HbAS to P. falciparum malaria could be mediated by the reduced ability of parasites to grow and multiply in HbAS cells, due to high red cell membrane resistance to the invading parasites and a hypoxic environment within the red cell which inhibit their development.Citation11,Citation12 It could also be by their early removal from the circulation, through various mechanisms, including the ability of parasite-infected HbAS erythrocytes to get sickled six times more readily than non-parasitized HbAS cellsCitation13,Citation14 which may lead to intracellular parasite deathCitation15 and/or their enhanced removal by the accelerated acquisition of malaria-specific immunity; both innateCitation16,Citation17 and acquired immunity to the parasite.Citation18,Citation19 Anaemia is known to be a prominent feature of falciparum malaria, usually with higher prevalence in HbAA-infected children than HbAS. The higher haemoglobin concentration in HbAS-infected children has been attributed to protection against malaria-induced anaemia.Citation4,Citation6 Normally, the number of reticulocytes in the blood indicates the degree of activity of the bone marrow in the production of erythrocytes, and when the marrow is very active (as in anaemia) their number increases. In the study, the presence of high malaria parasitaemia level in HbAA is suspected to have reduced the reticulocyte count, supporting previous studies.Citation20,Citation21 This is attributed to the dyserythropoietic effect emanating from the falciparum parasitaemia, possibly, through the malarial pigment or haemozoin,Citation22 some oxidized products, or some imbalance in cytokine response, exerting some negative impact on erythropoiesis.Citation20
During P. falciparum infection, there are changes in leukocyte proliferation and function. In this study the significant changes in white blood cells, like leukocytosis, monocytosis, and immature granulocytosis (shift to the left were prominent in infected HbAA children than HbAS. The observation is not uncommon in falciparum malaria.Citation23,Citation24 Leukocytosis may be associated with severity of malaria and adverse outcome.Citation25 Monocytosis is a major leukocytic change associated with malaria infection and also important in developing immunity against falciparum malaria. The monocytes are supposed to act against the parasites via several mechanisms, including phagocytosis of parasite-infected red blood cells, and release of cytokines,Citation26 and antibody-dependent cellular inhibition of parasite growth.Citation27 Immature granulocytosis could be attributed to the rapid release of marrow granulocyte precursor's into the blood (creating apparent neutropenia, due to changes in intravascular granulocytes distribution, coupled with a shift of neutrophils from the circulating to the marginated cell pools.Citation28
Platelet abnormalities are well-known complication in malaria. The platelet count was lower in HbAA-infected children. The association of thrombocytopaenia and malaria in children has previously been described,Citation29 although very common, was not associated with adverse outcome.Citation25 The mechanism of thrombocytopaenia in malaria is still not well understood but direct peripheral destruction, excessive removal of platelets by splenic poolingCitation30 as well as platelet consumption by the process of disseminated intravascular coagulopathyCitation31 have been postulated. ESR of malarial-infected children was higher than normal children and ESR of HbAA with higher parasitaemia was higher than that of HbAS with low parasitaemia level. This is believed to be due to the increase of immunoglobulin concentrations in malaria patients, which in turn, affects the roleaux formation of erythrocytes.Citation32
The effect of malaria on iron metabolism is not fully understood, but distinct changes in iron metabolism occur during malaria infection which may affect iron status assessment. The disturbances in iron homeostasis can result in the development of hypoferraemia, a high rise in serum ferritin concentrations and impaired iron incorporation in haemoglobin.Citation33 Malaria-induced hepcidin (the iron-regulatory hormone) expression provides mechanistic explanation for the observed maldistribution of iron in patients with malaria, as its expression leads to decreased iron bioavailability for erythropoiesis.Citation34 The release of hepcidin, induced by some cytokines, like IL-6, causes increased serum ferritin, accounting for the reduced bioavailability of iron.Citation35
The interactions involving haemoglobinopathy, malaria, and iron status are complex. In this study, iron status parameters, including serum iron, ferritin, and transferrin saturation were significantly lower in HbAS-infected children than HbAA. Iron-deficiency anaemia appears to develop more slowly in HbAS malaria parasite-infected children, as they seem protected against haematological complications of malaria. It has been reported that iron supplementation to pregnant women with HbAS increased their susceptibility to malaria.Citation36 In this context, the study corroborates another report.Citation37 that iron deficiency might play some role in the mechanism through which children with sickle cell trait are protected against malaria.
In conclusion, infection with P. falciparum malaria caused significant changes in haematological parameters of HbAA children, compared to HbAS children, showing the latter to be less affected by the infection. The findings of this study support the observation that the presence of HbAS seems to be protective against malaria haematological alterations.
What is new in our study is that we have demonstrated the protective effect of HbAS, using parasite densities, the various blood cellular components (white cells, platelets) and some red cell indices and measures of iron markers (ferritin, iron, TIBC, transferrin saturation). Other studies had used fewer parameters like parasite densities and anaemia severity. For example, a study in Kenya,Citation38 they measured parasite density and haemoglobin levels.
The limitations of our study include the small sample size, the cross-sectional nature of the study, lack of knowledge of the malaria infectivity rates in the area of study and the possible non-uniformity in the exposure of the subjects to mosquitoes.
This study has important public health implications since malaria is one of the major causes of childhood morbidity and mortality around the globe. The prior adequate knowledge and awareness of Haemoglobin genotype of patient can prevent abnormal Haematological alterations associated morbidity in children. An improved understanding of how abnormal Haemoglobin genotype protect against malaria may provide important information required for the development of novel therapeutics in the future.
Authors’ contributions
A.A. designed the study and contributed in carrying out the clinical and laboratory work and interpreted and analysed the data, and drafted the paper. K.N. revised the paper, A.A. and K.N. read and approved the final manuscript and they are guarantors of the paper.
Acknowledgements
The authors are grateful to the parents, guardians, and children from the TurAlbaha District community for their participation in the study. They also thank Lahij Governorate Public Health Office and all the TurAlbaha District Hospital staff for their support during this study.
References
- WHO. WHO Global Malaria Program. World malaria report. Geneva: World Health Organization; 2010.
- Price RN, Simpson JA, Nosten F, Luxemburger C, Hkirjaroen L, ter Kuile F, et al. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65:614–22.
- Aidoo M, Terlouw DJ, Kolczak MS, McElroy PD, ter Kuile FO, Kariuki S, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 2002;359:1311–2.
- Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M, Snow RW, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–86.
- Cooke G, Hill A. Genetic of susceptibility to human infectious disease. Nat Rev Genet. 2001;2:967–77.
- McElroy PD, Lal AA, Hawley WA, Bloland PB, Kuile FO, Oloo AJ, et al. Analysis of repeated hemoglobin measures in full-term, normal birth weight Kenyan children between birth and four years of age. III: The Asembo Bay cohort project. Am J Trop Med Hyg. 1999;61:932–40.
- WHO. Manual of basic techniques for a health laboratory. 2nd ed. Geneva: World Health Organization; 2003.
- WHO. Nutritional anaemias: report of a WHO Scientific Group. Geneva: World Health Organization; 1968. Technical Report Series, No. 405. Available from: http://whqlibdoc.who.int/trs/WHO_TRS_405.pdf
- Nkuo-Akenji T, Wepngong P, Akoachere J. Effects of ABO/Rh blood group, G-6-P-D enzyme activity and haemoglobin genotypes on malaria parasitaemia and parasite density. Afr J Health Sci. 2004;11:93–7.
- Makani J, Komba AN, Cox SE, Oruo J, Mwamtemi K, Kitundu J, et al. Malaria in patients with sickle cell anemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood 2010;115:215–20.
- Friedman MJ. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci USA. 1978;75:1994–7.
- Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanisms for the protective effect of haemoglobin S against P. falciparum malaria. Nature 1978;274:701–3.
- Luzzatto L, Nwachuku-Jarrett ES, Reddy S. Increased sickling of parasitized erythrocytes as mechanism of resistance against malaria in the sickle-cell trait. Lancet 1970;1:319–21.
- Roth EF Jr, Friedman M, Ueda Y, Tellez I, Trager W, Nagel RL. Sickling rates of human AS red cells infected in vitro with Plasmodium falciparum malaria. Science 1978;202:650–2.
- Friedman MJ, Roth EF, Nagel RL, Trager W. Plasmodium falciparum: physiological interactions with the human sickle cell. Exp Parasitol. 1979;47:73–80.
- Hebbel RP. Sickle hemoglobin instability: a mechanism for malaria protection. Redox Rep. 2003;8:238–40.
- Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum-malaria in sickle-trait and beta thalassemia-trait. Blood 2004;104:3364–71.
- Abu-Zeid YA, Abdulhadi NH, Theander TG, Hviid L, Saeed BO, Jepsen S, et al. Seasonal changes in cell mediated immune responses to soluble Plasmodium falciparum antigens in children with haemoglobin AA and haemoglobin AS. Trans R Soc Trop Med Hyg. 1992;86:20–2.
- Le Hesran JY, Personne I, Personne P, Fievet N, Dubois B, Beyemé M, et al. Longitudinal study of Plasmodium falciparum infection and immune responses in infants with or without the sickle cell trait. Int Jo Epidemiol. 1999;28:793–8.
- Roberts DJ, Casals-Pascual C, Weatherall DJ. The clinical, pathophysiological features of malarial anaemia. Current Topics in Microbiology and Immunol. 2005;295:137–67.
- Barnwell JW. Malaria: death and disappearing erythrocytes. Blood 2006;107:854–6.
- Casals-Pascual C, Kai O, Newton CR, Peshu N, Roberts DJ. Thrombocytopenia in falciparum malaria is associated with high concentrations of IL-10. Am J Trop Med Hyg. 2006;75:434–6.
- Abdalla SH. Peripheral blood and bone marrow leucocytes in Gambian children with malaria: numerical changes and evaluation of phagocytosis. Ann Trop Pediatr. 1988;8:250–8.
- Reiley CG, Barrett O Jr. Leukocyte response in acute malaria. Am J Med Sci. 1971;262:153–8.
- Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Brit J Haematol. 2002;119:839–47.
- Nielson H, Theander TG. Blood monocyte oxidative burst activity in acute P. falciparum malaria. Acta Pathol Microbiol Immunol Scand. 1989;97:469–71.
- Tebo AE, Kremsner PG, Luty AJ. Plasmodium falciparum: a major role for IgG3 in antibody-dependent monocyte-mediated cellular inhibition of parasite growth in vitro. Exp Parasitol. 2001;98:20–8.
- Dale DC, Wolf SM. Studies of the neutropenia of acute malaria. Blood 1973;41:197–206.
- Gérardin P, Rogier C, Ka AS, Jouvencel P, Brousse V, Imbert P. Prognostic value of thrombocytopenia in African children with falciparum malaria. Am J Trop Med Hyg. 2002;66:686–91.
- Skudowitz RB, Katz J, Lurie A, Levin J, Metz J. Mechanisms of thrombocytopenia in malignant tertian malaria. Brit Med J. 1973;2:515–8.
- Essien EM. The circulating platelet in acute malaria infection. Brit J Haematol. 1989;72:589–90.
- Eriksson B, Hellgren U, Rombo L. Changes in erythrocyte sedimentation rate, C-reactive protein and hematological parameters in patients with acute malaria. Scand J Infect Dis. 1989;21:434–41.
- Stoltzfus RJ, Chwaya HM, Albonico M, Schulze KJ, Savioli L, Tielsch JM. Serum ferritin, erythrocyte protoporphyrin and hemoglobin are valid indicators of iron status of school children with malaria in a malaria holoendemic population. J Nutr. 1997;127:293–8.
- de Mast Q, Nadjm B, Reyburn H, Kemna EH, Amos B, Laarakkers CM, et al. Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J Inf Dis. 2009;199:253–62.
- Weiss G. Iron metabolism in the anemia of chronic disease. Biochim BiophysActa. 2008;1790:682–93.
- Menendez C, Todd J, Alonso PL, Francis N, Lulat S, Ceesay S, et al. The response to iron supplementation of pregnant women with the haemoglobin genotype AA or AS. Trans R Soc Trop Med Hyg. 1995;89:289–99.
- Jeremiah ZA, Uko EK, Usanga EA. Relation of nutritional status, Sickle cell trait, glucose-6-phosphate dehydrogenase deficiency, iron deficiency and asymptomatic malaria infection in the Niger Delta, Nigeria. J Med Sci. 2008;8:269–74.
- Otieno W, Estambale BBA, Aluoch JR, Gondi SMO, Stoute JA. Association between sickle cell trait and low density parasitaemia in a Plasmodium falciparum holoendemic region of western Kenya. Intl J Trop Dis Health. 2012;2:231–40