Abstract
Objectives
The aim of this report was to investigate the tailored treatment strategies for isolated central nervous system (CNS) recurrence in adult patients with acute myeloid leukemia (AML).
Methods
Isolated CNS recurrence was documented in 34 patients: there were 18, 6, and 10 patients with meningeal involvement type (type A), cranial nerve palsy type (type B), and myeloid sarcoma type (type C), respectively. For patients with type A, intrathecal chemotherapy was the predominant strategy. For type B, systemic HD-Ara-C with four cycles was the main treatment. For type C, cranial irradiation or craniospinal irradiation was adopted and two cycles of HD-Ara-C were given after the irradiation.
Results
The 5-year cumulative incidence of CNS recurrence was 12.8%. There was a significantly higher WBC count (32.6∼60.8 × 109/l) in patients at first diagnosis who developed CNS recurrence (all of the three types) compared with patients with no CNS recurrence (10.1 × 109/l) (P = 0.005). We found that a significantly more patients with AML-M5 and 11q23 abnormalities developed CNS recurrence in type A (P < 0.001, 0.005). Twenty-four out of 34 patients (70.6%) with CNS recurrence achieved CNS complete remission at a median of 58 days (range, 30–120). The 3-year disease-free survival and overall survival estimates for all CNS recurrence patients were 21.6 and 25.3%, respectively.
Discussion
This report indicates that the tailored CNS-directed strategy is an effective modality to treat CNS recurrence in adult AML, but further studies are needed to improve the long-term survival.
Introduction
Central nervous system (CNS) recurrence in adult acute myeloid leukemia (AML) is generally associated with a very poor prognosis.Citation1–Citation3 The prevalence of and risk factors for CNS recurrence in adult patients with AML have been analyzed recently in two clinical studies.Citation2,Citation3 However, the limited data about treatment of CNS recurrence in adult AML are derived from studies of acute lymphoblastic leukemiaCitation4,Citation5 or pediatric AML,Citation6,Citation7 and the therapies used on the patients in previous reported clinical studies varied from intrathecal (IT) chemotherapy only, to high-dose systemic chemotherapy or CNS radiotherapy. There is no consensus as to which therapy should be used in CNS recurrence of adult AML patients, and there is no therapy that proved to be more effective than the other. In this article, we will investigate the diagnostic criteria, risk factors, and tailored treatment of isolated CNS recurrence at first relapse in patients with AML at a single institution, emphasizing new findings which could be benefit to improve the prognosis.
Patients and methods
This retrospective study included all adult patients over 18 years of age who were diagnosed with non-promyelocytic AML in Anhui provincial hospital between September 1999 and January 2010.
Induction and consolidation chemotherapy
Each patient received at least one course of induction chemotherapy with DA protocol (daunorubicin 45 mg/m2 daily for 3 days, and cytarabine 150 mg/m2 daily for 7 days). Consolidation therapy was administered after the achievement of complete remission (CR) as the following: high doses cytarabine (HD-Ara-C) (1.5–2.0 g/m2 every 12 h for 3 days) with four courses; or sequential therapy of DA, HA (homoharringtonine 2 mg/m2 daily for 7 days, and cytarabine 150 mg/m2 daily for 7 days), and MA (mitoxantrone 8 mg/m2 daily for 3 days, and Ara-C 150 mg/m2 daily for 7 days) with three cycles. Patients who achieved CR and had a five or six antigen HLA-matched family donor may be allocated to allogeneic hematopoietic stem cell transplantation (HSCT).
All patients did not receive any CNS prophylaxis during their induction and consolidation treatment. Patient with induction treatment failure or CNS involvement at the time of initial diagnosis was excluded from this study.
Classification of CNS leukemia
Isolated CNS recurrence was defined as relapse in the CNS with no evidence of bone marrow involvement within 30 days of the CNS disease. According to cerebrospinal fluid (CSF) findings, neurologic symptoms and signs, and CNS imaging examinations, we divided CNS leukemia into three types: meningeal involvement type, cranial nerve palsy type, and myeloid sarcoma (MS) type. Meningeal involvement type, which was also called meningeal leukemia, was identified by CSF with five or more WBC/μl and cytospin positive for blasts without cranial nerve palsy or mass effect in CNS. Cranial nerve palsy type was a form of palsy involving one or more of the cranial nerves, most of which were facial nerve paralysis, due to infiltration by leukemia cells. MS type was diagnosed by imaging tests such as computed tomography (CT) or magnetic resonance imaging (MRI), which showed the nonhemorrhagic mass effect in the brain or spinal cord, or by histopathology according to mass aspiration or biopsy. Patients with cranial nerve palsy or patients with a cerebral mass in combination with blasts in CSF after cytocentrifugation were regarded as cranial nerve palsy type or MS type.
Statistical considerations
CNS-CR was defined as a complete clearance of blasts from the CSF at least on two consecutive examinations in a week, relief of neurological symptoms, and disappearance of CNS mass by CT or MRI. CNS second recurrence was defined as the reappearance of blasts in CSF or development of clinical signs of CNS leukemia (facial nerve palsy, brain/eye involvement) after achievement of a CNS-CR. CNS refractory disease was defined as failure to achieve a CNS-CR at the end of CNS-directed treatment. Cox multivariate regression analysis was applied to identify the high-risk factors associated with poor survival outcome, and the variables were compared by using Student's t tests or χ2 tests, as appropriate. Disease-free survival (DFS) and overall survival (OS) after relapse was estimated by the Kaplan–Meier method, and subgroup comparisons were performed by the log-rank test. All analyses were performed with SPSS (version 17.0).
Results
Patient characteristics
Two hundred and ninety-nine of 432 adult patients with a diagnosis of non-promyelocytic AML undergoing induction chemotherapy achieved first CR. Sixty-eight patients received four courses with HD-Ara-C and 231 patients received three cycles of sequential therapy as consolidation treatment. Only 13 patients received allogeneic HSCT and 6 patients received autologous HSCT during the first period of CR.
All patients with evidence of CNS recurrence underwent a bone marrow examination at that time. A bone marrow recurrence within 30 days from the detection of CNS disease was considered to be concurrent with systemic recurrence. Isolated CNS recurrence was documented in 34 patients: there were 18, 6, and 10 patients with meningeal involvement type (type A), cranial nerve palsy type (type B), and MS type (type C), respectively. The median time to CNS recurrence was 6 months (range, 1–37 months), and the 5-year cumulative incidence of CNS recurrence was 12.8%. The data for these 34 patients compared with the 265 patients who entered CR after induction therapy but did not develop a CNS recurrence are listed in .
Table 1. CNS recurrence patients’ characteristics at the time of diagnosis compared with patients without CNS recurrence
Risk factors of CNS recurrence
The median age of patients at diagnosis who developed an isolated CNS recurrence was 35.7 years (range, 18–65 years). There was a significantly higher WBC count (32.6∼60.8 × 109/l) in patients at first diagnosis who went on to develop CNS recurrence (all of the three types) compared with patients with no CNS recurrence (10.1 × 109/l) (P = 0.005). In comparing FAB subtypes and cytogenetic abnormalities of the patients with CNS recurrence versus no CNS recurrence, we found that a significantly more patients with AML-M5 and 11q23 abnormalities developed CNS recurrence in type A (P < 0.001, 0.005). There was a lack of correlation between FAB subtypes or cytogenetic abnormalities and CNS recurrence of cranial nerve palsy or MS type. There were no associations between CNS relapse and the LDH level, bulk disease, leukemia cutis, and the consolidation treatment protocols.
Tailored CNS-directed treatment and outcome
All patients underwent routine diagnostic lumbar puncture for CSF sampling and concomitantly received IT cytarabine (50 mg) plus dexamethasone (5 mg) once CNS recurrence was suspected.
For different types of CNS leukemia, we gave the different CNS-directed treatment strategies (). For patients of type A, IT chemotherapy was the predominant strategy. All of these patients received IT chemotherapy with methotrexate (MTX) (10 mg) plus dexamethasone (5 mg) alternating with cytarabine (50 mg) twice weekly until the findings disappeared on two consecutive CSF examinations, and then given monthly for a minimum of five IT administrations. Eight patients received further two cycles of HD-Ara-C and craniospinal irradiation (CSI) (20 Gy) because of CNS refractory disease (n = 3) or CNS second recurrence (n = 5). Six patients who had CNS recurrence with type B received systemic HD-Ara-C with four cycles, and alternating IT-MTX or cytarabine was also administered in four patients due to the blasts in CSF which were detected at the time of routine diagnostic lumbar puncture. For patients of type C, cranial or spinal irradiation (24 Gy) was adopted according to the mass localization, and two cycles of HD-Ara-C were given after the irradiation. Short-course high-dose methylprednisolone (HDMP) treatment (1 g/day for 3 days, 500 mg/day for 2 days) was used at the same time when the mass effect in the imaging was obvious (n = 2) or the patient presented the symptoms of acute spinal cord compression (n = 2).
Figure 1. Tailored CNS-directed treatment strategies and the treatment outcome. CNS, central nervous system; CNSL, central nervous system leukemia; IT-MTX, intrathecal-methotrexate; Ara-C, cytarabine; CSI, cerebrospinal irradiation; HDMP, high-dose methylprednisolone; CNS-CR, CNS complete remission; DFS, disease-free survival; OS, overall survival. All patients underwent routine diagnostic lumbar puncture for CSF sampling and concomitantly received intrathecal (IT) cytarabine (50 mg) plus dexamethasone (5 mg) once CNS recurrence was suspected. CNS refractory disease is defined as failure to achieve a CNS-CR at the end of CNS directed treatment. CNS second recurrence is defined as the reappearance of blasts in CSF or development of clinical signs of CNS leukemia (e.g. facial nerve palsy, brain/eye involvement) after achievement of a CNS-CR.
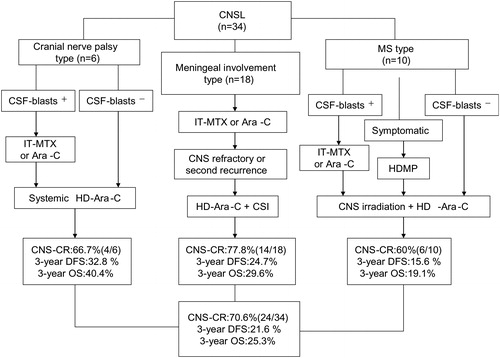
Overall, 24 out of 34 patients (70.6%) with CNS recurrence achieved CNS-CR at a median of 58 d (range, 30–120). But unfortunately, 10 patients died due to CNS refractory disease, and 15 patients died due to CNS second relapse (n = 3) or bone marrow relapse (n = 6) or both (n = 6) in the end. The median OS for the entire group of 34 patients with CNS leukemia (CNSL) was 6.64 months (range, 1–47 months). The 3-year DFS and OS estimates for all CNS recurrence patients were 21.6 and 25.3%, respectively (details in ).
Discussion
In this study, we confirmed the previously reported association between CNS recurrence and high WBC count at diagnosis.Citation3,Citation7 These reports also indicated that patients with AML and CNS disease were associated with chromosome 16 inversion and chromosome 11 abnormalities at diagnosis.Citation3,Citation7 We found that CNS recurrence of meningeal involvement type, but not of cranial nerve palsy or MS type, was associated with FAB M5 subtype and the cytogenetic abnormality 11q23. Martínez-Cuadrón’ study showed an extremely low incidence of meningeal relapse in adult patients with AML treated with modern protocols including stem cell transplantation, and the author hypothesized that modern chemotherapies including frequent stem cell transplantation might reduce the risk of CNS relapse by eliminating the residual leukemic cells in the CNS.Citation2 However, the treatment protocol was not confirmed to be an independent risk factor in our study, and we did not find any correlation between CNS recurrence and HD-Ara-C group or sequential therapy group in the consolidation treatment period. The 5-year cumulative incidence of CNS relapse (12.8%) in our study was higher than what previously reported (1.3–11%),Citation2,Citation8 perhaps due to the limited number of patients received HSCT.
The treatment of CNS recurrence of AML often poses some therapeutic dilemmas, e.g. CNS recurrence includes a wide variety of clinical presentations which should be treated with different CNS-directed treatment strategies,Citation8,Citation9 and CNS overtreating, which is difficult to avoid, could lead to the severe CNS toxicities, such as neurocognitive deficits, endocrinopathy, and second cancers.Citation10 How to provide effective CNS therapy and minimize neurotoxicity and late side effects are major problems encountered in our clinical practice. In this study, we divided CNS recurrence into three types (meningeal involvement type, cranial nerve palsy type, and MS type) according to the different pathophysiological characteristics of CNSL, and then gave the tailored CNS-directed treatment strategies for the different group patients.
Meningeal leukemia results from the infiltration of blood leukemia cells to the leptomeninges (the arachnoid membrane and the pia mater), and the CSF, which flows in the subarachnoid space between the pia and the arachnoid, may then provide a route for metastasis along the entire neuraxis. IT drug administration, which is the effective strategy for this type,Citation11 may produce cytotoxic concentrations directly to kill blasts in the CSF even when small doses are used, and systemic toxicity is very rare. If IT treatment efficacy is poor (CNS refractory or CNS second relapse), HD-Ara-C and CSI should be used. In our study, CNS-CR was obtained in 14 (77.8%) of 18 patients in this group.
As a result of blasts infiltration and inflammation of the cranial nerve, nerve swelling and compression in the narrow bone canal are thought to lead to neural signals block and nerve inhibition or damage, and this is perhaps the right pathophysiology of cranial nerve palsy. From the perspective of anatomy, IT chemotherapy agents cannot reach the nerve distribution area, and cranial radiotherapy cannot confine to the nerve fibers only and maybe cause damage of normal brain tissue. High-dose systemic administration may produce more uniform drug distribution throughout the cranial nerves, and this treatment modality was associated with CNS-CR rate of 66.7% (4 of 6) in this group.
MS type is a solid collection of leukemic cells occurring in the cranial or spine, and radiation therapy is a principal modality in the treatment of CNS mass. We used two cycles of HD-Ara-C before radiotherapy because it can achieve therapeutic levels in the CSF and may penetrate into the the core of the tumor. Two large retrospective studies on primary CNSL have not demonstrated benefits from adding IT drug delivery in patients treated with HD-MTX.Citation12,Citation13 Glucocorticoids at high doses have been shown to induce differentiation and apoptosis of myeloid leukemic cells in vitro studies.Citation14,Citation15 Based on these findings, we often used HDMP to reduce inflammatory swelling and lysis leukemia blasts if the patient presented the symptoms of acute spinal cord compression or the mass effect was obvious in the imaging. Ten patients with this type were treated with CNS irradiation plus HD-Ara-C and four patients received HDMP at the same time, and the CNS-CR was obtained in 6 (60%) of 10.
To the best of our knowledge, this is the first report to identify the appropriate CNS recurrence group patients and to evaluate the effectiveness of the tailored CNS-directed treatment modalities. Our results indicated that treatment with the tailored CNS-directed strategies could result in a relative high CNS-CR rate (total of 70.6%), but the long-term survival was still dismal: the median OS was only 6.64 months, and 10 patients died due to CNS refractory disease, and 15 patients died due to CNS second relapse and/or bone marrow relapse. The dismal long-term outcome of this study which was consistent with previously reported dataCitation4,Citation5 stated the need for more intensified systemic therapy to reduce the risk for systemic relapse. However, this is a non-prospective and non-randomized study, and the number of patients is small; further study of the therapeutic protocols, such as high-dose chemotherapy combined with HSCT, are needed to establish the most effective treatment modality to treat the CNS recurrence in adults with AML.
Funding
This work was supported by National Science Foundation (81250001), Ministry of Health funding for public service research and special projects (201202017).
Acknowledgements
C. Z. designed the study, analyzed the data, and wrote the paper. All other authors performed the research, edited the paper, and contributed to analysis of research.
References
- Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115:453–74.
- Martínez-Cuadrón D, Montesinos P, Pérez-Sirvent M, Avaria A, Cordón L, Rodríguez-Veiga R, et al. Central nervous system involvement at first relapse in patients with acute myeloid leukemia. Haematologica 2011;96:1375–9.
- Shihadeh F, Reed V, Faderl S, Medeiros LJ, Mazloom A, Hadziahmetovic M, et al. Cytogenetic profile of patients with acute myeloid leukemia and central nervous system disease. Cancer 2012;118:112–7.
- Surapaneni UR, Cortes JE, Thomas D, O'Brien S, Giles FJ, Koller C, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia. Cancer 2002;94:773–9.
- Sancho JM, Ribera JM, Oriol A, Hernandez-Rivas JM, Rivas C, Bethencourt C, et al. Central nervous system recurrence in adult patients with acute lymphoblastic leukemia: frequency and prognosis in 467 patients without cranial irradiation for prophylaxis. Cancer 2006;106:2540–6.
- Abbott BL, Rubnitz JE, Tong X, Srivastava DK, Pui CH, Ribeiro RC, et al. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: a single institution's experience. Leukemia 2003;17:2090–6.
- Johnston DL, Alonzo TA, Gerbing RB, Lange BJ, Woods WG. Risk factors and therapy for isolated central nervous system relapse of pediatric acute myeloid leukemia. J Clin Oncol. 2005;23:9172–8.
- Stewart DJ, Keating MJ, McCredie KB, Smith TL, Youness E, Murphy SG, et al. Natural history of central nervous system acute leukemia in adults. Cancer 1981;47:184–96.
- Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukemia. Lancet Oncol. 2008;9:257–68.
- Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–9.
- Matloub Y, Lindemulder S, Gaynon PS, Sather H, La M, Broxson E, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children's Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children's Oncology Group. Blood 2006;108:1165–73.
- Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology 2002;58:1513–20.
- Khan RB, Shi W, Thaler HT, DeAngelis LM, Abrey LE. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol. 2002;58:175–8.
- Hiçsönmez G, Cetin M, Aslan D, Ozyürek E. The role of short course of high-dose methylprednisolone in children with acute myeloblastic leukemia (FAB M2) presented with myeloid tumor. Pediatr Hematol Oncol. 2003;20:373–9.
- Hiçsönmez G. The effect of steroid on myeloid leukemic cells: the potential of short-course high-dose methylprednisolone treatment in inducing differentitation, apoptosis and in stimulating myelopoiesis. Leuk Res. 2006;30:60–8.
