Abstract
Objectives
Currently, multiple myeloma (MM) is an incurable disease. Despite the fact that arsenic trioxide (ATO) shows promising results in vitro, data from treatment of patients with MM are disappointing. Due to these discrepancies, we compared the efficacy and selectivity of ATO at two different concentrations in samples from MM patients.
Methods
The extent of apoptosis induced by 2 and 5 µM ATO was evaluated by flow cytometry using annexin V. 34 diagnostic bone marrow samples obtained from MM patients were analysed.
Results
5 µM ATO efficiently induced apoptosis in primary samples. Besides efficacy, also selectivity of action on MM cells in comparison to remaining haematopoietic cells was demonstrated for 5 µM ATO but not for 2 µM ATO.
Discussion
Our study on primary samples confirmed that ATO has a potential role in therapeutic management of MM. Further controlled studies on MM patients are needed.
Introduction
Arsenic trioxide (ATO) has antitumor activity in a variety of haematological malignancies and against cancer cells of solid tumour origin.Citation1,Citation2 It represents a promising agent in the treatment of multiple myeloma (MM) since it targets MM cells and the bone marrow microenvironment, known to be involved in the disease pathogenesis. Various potential mechanisms of action have been described for ATO, and the rationale for using ATO in MM is based on its multifaceted effects.Citation3–Citation6 Importantly, pharmacological concentrations of ATO preferentially induce apoptosis in MM cells while sparing most normal myeloid cells.Citation7
However, there are still discrepancies about the in vitro and in vivo efficacy of ATO in the treatment of MM. While some studies have demonstrated the efficacy of ATO in vitro,Citation4,Citation8 others claim that resistance to ATO treatment is relatively common.Citation9,Citation10 The discrepancies about its efficacy in vitro may partly arise from the fact that different methods were applied in order to detect MM cells in a complex population of bone marrow cells.Citation4,Citation8,Citation11 The discrepancies concerning its efficacy in vivo may be due to considerable methodological flaws as described elsewhere.Citation12 Thus, further controlled studies are needed to confirm the efficacy of ATO in the treatment of MM patients.
In order to address these discrepancies, we measured the extent of apoptosis induced by ATO in primary diagnostic samples from MM patients. In MM cells, ATO exerts antimyeloma effects at clinically achievable concentrations (2–5 µM).Citation4 We compared its efficacy and selectivity at two different clinically achievable concentrations on MM cells and the remaining haematopoietic cells in samples from MM patients.
Methods
Primary MM cells: Bone marrow specimens were collected after obtaining each patient's informed consents. The study comprised 34 newly diagnosed MM patients, 25 males and 9 females. All MM patients included in the study had an aberrant immunophenotype at the time of diagnosis that was confirmed by a combination of the following monoclonal antibodies: CD38, CD138, CD45, CD56, CD19, CD20, CD117, and CD28.
Bone marrow cells were cultivated overnight (MarrowMAX, Gibco, Carlsbad, CA, USA) at 37°C and 5% CO2. After 24 hours of cultivation, the cells were distributed into wells at a final concentration of 1 × 106 cells/ml. Stock ATO (Sigma-Aldrich, Wien, Austria) solution was prepared as 0.1% As2O3 in distilled water. ATO was added to the growth medium to a final concentration of 2 or 5 µM. Cells incubated with camptothecin, a known inductor of apoptosis,Citation13 at a final concentration 50 µM, represented a positive control, while those with nothing added served as a negative control. The incubation lasted up to 48 hours. In all experiments performed, apoptosis in the positive control was well above that in the negative control.
Sample preparation for apoptosis measurement: The cells were harvested, lysed with 0.15 M NH4Cl, washed in phosphate buffered saline, and incubated with CD38 (PC5-conjugated, I) and CD138 (PE-conjugated, I) antibodies. The extent of apoptosis was evaluated using annexin V (Annexin V-FITC Kit, BC) according to the manufacturer's instructions. The extent of MM cell apoptosis was measured and analysed by flow cytometry (FC500, BC). Since the emission spectra of fluorescent dyes used in this study overlap, appropriate colour compensation was performed. Apoptosis in MM cells and normal haematopoietic cells was determined as previously reported.Citation1,Citation14 With the use of a specific gating strategy, the annexin V/PI histogram was gated on the plasma cell population (CD38 +/CD138+) with subsequent exclusion of cellular debris (red events, ). This gating strategy enabled us to focus specifically on MM plasma cell apoptosis (blue events, ). Blue and green events that were Annexin V-/PI- were considered as live MM plasma cells and live other haematopoietic cells, respectively.
Statistical analysis: Statistical evaluation of the extent of apoptosis and selectivity of ATO for MM cells was conducted using one way repeated measures analysis of variance.
Results
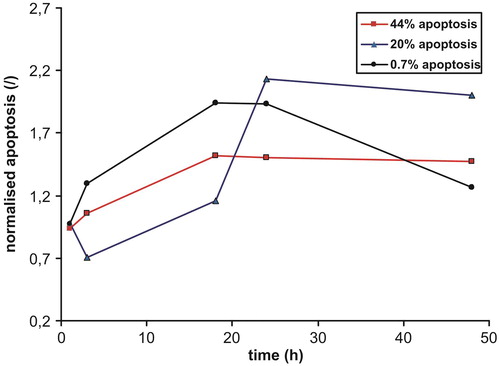
The incubation of bone marrow samples with ATO was started after 24 h of precultivation in growth medium. Primary bone marrow samples differed considerably in plasma cell infiltration rate (0.5–53.3%; average 15.6%) and spontaneous apoptosis of MM cells (4–91%; average 39%). The latter is referred to as MM cell apoptosis in the negative control. Time dependence experiments were done in primary samples to determine an optimal incubation time with ATO. The extent of apoptosis was evaluated after 1, 3, 18, 24, and 48 hours of incubation with 5 µM ATO. Three samples that differed markedly in spontaneous MM cell apoptosis (0.7, 20, and 44%) were selected. The extent of apoptosis was normalized to the spontaneous apoptosis of each sample. The time courses were similar in all three experiments, the maximal value or plateau being reached after 18–24 hours of incubation ().
Figure 1. Time dependence of the extent of MM cell apoptosis in three different primary samples. Values were normalized to the negative control.
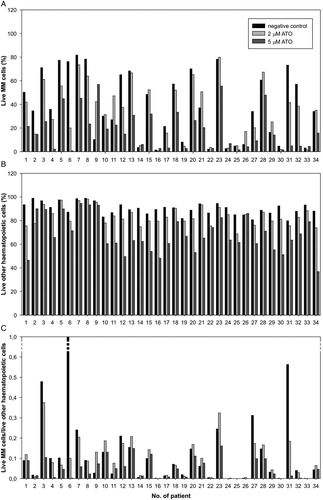
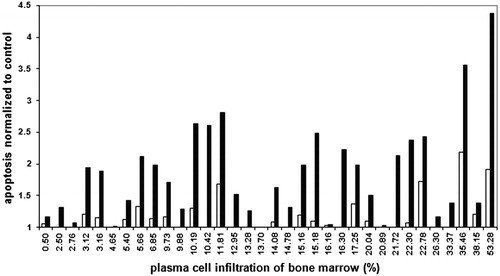
We analysed the extent of apoptosis induced by 2 and 5 µM ATO in 34 diagnostic bone marrow samples after 24 hours of incubation. Since apoptosis was intensive already in the negative control, we normalized the values of apoptosis induced by ATO to the extent of apoptosis in the negative control for the same sample. The normalized values for both ATO concentrations, presented in relation to the proportion of MM cells in the primary bone marrow samples are shown in . The extent of apoptosis induced by 5 µM ATO was significantly higher than that of the control (P < 0.001) while the extent of apoptosis induced by 2 µM ATO was not (P = 0.233) ().
Figure 2. Extent of MM cell apoptosis normalized to the negative control. Empty bars, 2 µM ATO; black bars, 5 µM ATO.
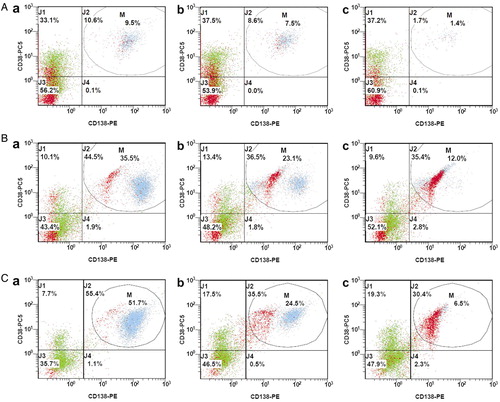
The consequence of apoptosis induced by ATO is a reduction of MM cells in the sample. The three representative cases that differed considerably in initial MM plasma cell infiltration rate showing a stepwise decline in MM cell proportion and increasing apoptosis from the negative control to 2 µM ATO and 5 µM ATO are presented in .
Figure 3. Decrease in the proportion of MM cells after 24 hours of incubation with ATO. Blue/light grey events, MM cells; red/dark grey events, cell debris; a, negative control; b, 2 µM ATO; c, 5 µM ATO. A, MM plasma cell apoptosis for patient no. 16: a = 24.3; b = 18.7; c = 63.3; B, MM plasma cell apoptosis for patient no. 31: a = 26.6; b = 58.0; c = 94.5; C, MM plasma cell apoptosis for patient no. 6: a = 22.2; b = 42.5; c = 97.3.
To evaluate the toxicity of ATO to haematopoietic cells and the selectivity of its action on MM plasma cells, we determined the proportion of cells for both populations that were still alive after 24 hours of incubation with ATO (). In comparison to the control, an average reduction of other haematopoietic cells by 7% and 25% was observed for 2 µM ATO and 5 µM ATO, respectively, confirming that ATO toxicity is not limited to MM plasma cells. In order to evaluate the selectivity of the action of ATO on MM cells, we calculated the ratio between live MM cells and live other haematopoietic cells. When compared to the negative control, this ratio was significantly reduced for 5 µM ATO (P < 0.05) but not for 2 µM ATO (P > 0.05) (C).
Discussion
There is a lack of data on MM cell apoptosis induced by ATO or other agents in diagnostic samples. This can be partially explained by difficulties in obtaining a suitable amount of primary MM cells. We analysed samples from patients undergoing a routine diagnostic workup for MM. Material obtained by bone marrow aspiration is often non-representative due to few bone marrow cells being aspirated and/or because of substantial contamination with peripheral blood.Citation15 Our samples differed considerably in infiltration rate, which was less than 10% in approximately one third of them (). The removal of bone marrow cells from their microenvironment represents a stress for the cells. To achieve their adaptation to the conditions ‘in vitro’, 24 hours pre-cultivation was done before the apoptosis induction experiments were started. In the meantime cytomorphological evaluation of the bone marrow smear was performed, and the aberrant immunophenotype of MM cells was confirmed by flow cytometry. Only in samples in which the diagnosis of MM had been established, apoptosis induction by ATO was initiated. Spontaneous apoptosis (as determined in the negative control) was variable, but it was pronounced in the majority of cases. To exclude the influence of spontaneous apoptosis, we normalized the values of induced apoptosis to those of the negative control ().
There is a diversity of literature data on clinically achievable concentrations of ATO, with the reported values ranging from 1 to 10 µM.Citation9,Citation11,Citation16 In patients with acute promyelocytic leukaemia (APL), low concentrations of ATO (0.1–0.5 µM) induce differentiation of APL cells while higher concentrations (>0.5 µM) induce apoptosis.Citation17 In MM cells, ATO exerts antimyeloma effects at clinically achievable concentrations (2–5 µM).Citation4 We used ATO at concentrations of 2 and 5 µM to determine and compare its efficacy and selectivity.
The time course of biochemical events that accompany the morphological changes of apoptosis often varies widely depending upon a variety of factors, e.g. cell tissue, apoptosis-inducing agent, drug concentration, and exposure time.Citation18–Citation22 In order to empirically determine the time at which the exposure of phosphatidylserine on the outer membrane peaks, time-dependence experiments were performed. The extent of apoptosis induced in MM cells by 5 µM ATO reached the maximum after 18–24 hours of incubation independent of spontaneous apoptosis or infiltration rate (). With regard to this finding and due to technical feasibility, we measured the extent of apoptosis in all our experiments on 34 diagnostic samples after 24 hours of incubation with ATO, similarly as in the majority of experiments with MM cell lines.Citation23,Citation24 When compared to the negative control, the extent of apoptosis was significantly higher for 5 µM ATO (P < 0.001) but not for 2 µM ATO (P = 0.233) (). This can be explained by time and dose dependence,Citation25 considering that the extent of apoptosis at 2 µM ATO peaked only after 48 hours of incubation (data not shown), which is consistent also with previously published results.Citation26 Analysis in MM is frequently hindered by low infiltration of bone marrow by MM cells. Therefore, we analysed the apoptosis induction in relation to the infiltration rate (). At both tested ATO concentrations apoptosis was not dependent on the infiltration rate. It must be noted, however, that analysis of samples with a low infiltration rate is less reliable due to the small number of MM cells analysed. This number is further decreased after incubation with ATO (). ATO does not, however, induce apoptosis exclusively in MM plasma cells but also in other haematopoietic cells. Its toxicity (to haematopoietic cells) and selectivity (for MM cells) is shown in . Apoptosis is accompanied by complex morphological changes, which eventually lead to an increased amount of cellular debris and a decreased amount of live cells. Since this is a dynamic process which affects both MM cells and other haematopoietic cells, the ratio between the absolute number of cells in these two populations that are alive gives a more reliable evaluation of the selectivity of ATO than the proportion of live cells alone (C). Although the toxicity of ATO towards other haematopoietic cells is more pronounced at a concentration of 5 µM compared to 2 µM, its action is selective at the higher concentration.
It has been reported previously that the mechanisms of action of ATO on MM cells are relatively selective.Citation4,Citation8,Citation11 In our “ex vivo” experiments, neither efficacy nor selectivity of action on MM cells was confirmed for 2 µM ATO after 24 hours of exposure. On the other hand, both efficacy and selectivity of action on MM cells, in comparison to other haematopoietic cells, were demonstrated for 5 µM ATO. It should be pointed out, however, that differences in apoptosis between individual samples were prominent. We believe that both spontaneous and ATO-induced apoptosis could be related to the stage of MM or some other prognostic factor (cytogenetics, achievement of remission).
Conclusion
Herein, we demonstrated that short-term exposure of MM primary cells to ATO is more efficient when the agent is used at a higher concentration. Our experiments also showed that this action of ATO is selective. We can conclude that ATO represents a promising agent in the treatment of MM. However, further controlled studies are needed to confirm these findings before ATO can be incorporated in the therapeutic management of patients with MM.
Acknowledgement
The work was supported by Slovenian Research Agency (ARRS P3-0289).
References
- Ge F, Lu XP, Zeng HL, He QY, Xiong S, Jin L, et al. Proteomic and functional analyses reveal a dual molecular mecanism underlying arsenic-induced apoptosis in human multiple myeloma cells. J Proteome Res. 2009;8:3006–19.
- Gazitt Y, Akay C. Arsenic trioxide: an anti cancer missile with multiple warheads. Hematology 2005;10(3):205–13.
- Harousseau JL, Shaughnessy J, Richardson P. Multiple myeloma. Hematology 2004;237–56.
- Hayashi T, Hideshima T, Akiyama M, Richardson P, Schlossman RL, Chauhan D, et al. Arsenic trioxide inhibits growth of human multiple myeloma cells in the bone marrow microenvironment. Mol Cancer Ther. 2002;1:851–60.
- Piazza FA, Gurrieri C, Trentin L, Semenzato G. Towards a new age in the treatment of multiple myeloma. Ann Hematol. 2007;86:159–72.
- Redzepovic J, Weinmann G, Ott I, Gust R. Current trends in multiple myeloma management. J Int Med Res. 2008;36:371–86.
- Munshi NC. Arsenic trioxide: An emerging therapy for multiple myeloma. Oncologist 2001;6(2):17–21.
- Rousselot P, Labaume S, Marolleau JP, Larghero J, Noguera MH, Brouet JC, et al. Arsenic trioxide and melarsoprol induce apoptosis in plasma cell lines and in plasma cells from myeloma patients. Cancer Res. 1999;59:1041–8.
- Wen J, Cheng HY, Feng Y, Rice L, Liu S, Mo A, Huang J, et al. P38 MAPK inhibition enhancing ATO-induced cytotoxicity against multiple myeloma cells. Br J Haematol. 2008;140:169–80.
- Zhou P, Kalakonda N, Comenzo RL. Changes in gene expression profiles of multiple myeloma cells induced by arsenic trioxide (ATO): possible mechanisms to explain ATO resistance in vivo. Br J Haematol. 2005;128:636–44.
- Grad JM, Bahlis NJ, Reis I, Oshiro MM, Dalton WS, Boise LH. Ascorbic acid enhances arsenic trioxide-induced cytotoxicity in multiple myeloma cells Blood 2001;98:805–13.
- Rollig C, Illmer T. The efficacy of arsenic trioxide for the treatment of relapsed and refractory multiple myeloma: A systematic review. Cancer Treat Rev. 2009;35(5):425–30.
- Smolewski P, Grabarek J, Lee BW, Johnson GL, Darzynkiewicz Z. Kinetics of HL-60 cell entry to apoptosis during treatment with TNF-α or camptothecin assayed by the stathmo-apoptosis method. Cytometry 2002;47:143–9.
- Reberšek K, Černelč P, Podgornik H. Evaluation of multiple myeloma cell apoptosis in primary bone marrow samples. Clin Lab. 2013;59(3–4):389–95.
- Smock KJ, Perkins SL, Bahler DW. Quantitation of plasma cells in bone marrow aspirates by flow cytometric analysis compared with morphologic assessment. Arch Pathol Lab Med. 2006;131(6):951–5.
- Bajenova O, Tang B, Pearse R, Feinman R, Childs BH, Michaeli J. RIP kinase is involved in arsenic-induced apoptosis in multiple myeloma cells. Apoptosis 2004;9:561–71.
- Lee C, Lin Y, Huang M, Lin C, Liu C, Chow J, et al. Increased cellular glutathione and protection by bone marrow stromal cells account for the resistance of non-acute promyelocytic leukaemia acute myeloid leukaemia cells to arsenic trioxide in vivo. Leuk Lymphoma 2006;47(3):521–9.
- Studzinski GP. Apoptosis: A practical approach. Oxford, UK: Oxford University Press, 1999.
- Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J Cell Mol Med. 2002;6(1):82–92.
- Härtel S, Zorn-Kruppa M, Tykhonova S, Alajuuma P, Engelke M, Diehl HA. Staurosporine-induced apoptosis in human cornea epithelial cells in vitro. Cytometry A. 2003;55(1):15–23.
- Riss TL, Moravec RA. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev Technol. 2004;2(1):51–62.
- Ramirez-Ortega M, Maldonado-Lagunas V, Melendez-Zajgla J, Carrillo-Hernandez JF, Pastelín-Hernandez G, Picazo-Picazo O, et al. Proliferation and apoptosis of HeLa cells induced by in vitro stimulation with digitalis. Eur J Pharmacol. 2006;534(1–3):71–6.
- Chauhan D, Singh AV, Ciccarelli B, Richardson PG, Palladino MA, Anderson KC. Combination of novel proteasome inhibitor NPI-0052 and lenalidomide trigger in vitro and in vivo synergistic cytotoxicity in multiple myeloma. Blood 2010;115(4):834–45.
- Morales AA, Gutman D, Cejas PJ, Lee KP, Boise LH. Reactive oxygen species are not required for an arsenic trioxide-induced antioxidant response or apoptosis. J Biol Chem. 2009;284(19):12886–95.
- Ravandi F. Arsenic trioxide: expanding roles for an ancient drug? Leukemia 2004;18:1457–9.
- Qu X, Du J, Zhang C, Fu W, Xi H, Zou J, Hou J. Arsenic trioxide exerts antimyeloma effects by inhibiting activity in the cytoplasmic substrates of histone deacetylase 6. PLoS One [e32215]. 2012;7(2). [ Accessed 2013 May 15] Available from:http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0032215.