Abstract
Objectives
To evaluate the influence of age on the relationships between biochemical and hematological variables and stability of erythrocyte membrane in relation to the sodium dodecyl sulfate (SDS) in population of 105 female volunteers between 20 and 90 years.
Methods
The stability of RBC membrane was determined by non-linear regression of the dependency of the absorbance of hemoglobin released as a function of SDS concentration, represented by the half-transition point of the curve (D50) and the variation in the concentration of the detergent to promote lysis (dD).
Results
There was an age-dependent increase in the membrane stability in relation to SDS. Analyses by multiple linear regression showed that this stability increase is significantly related to the hematological variable red cell distribution width (RDW) and the biochemical variables blood albumin and cholesterol.
Discussion
The positive association between erythrocyte stability and RDW may reflect one possible mechanism involved in the clinical meaning of this hematological index.
Keywords:
Introduction
Stability and fluidity are membrane properties that are essential to allow the cells to resist the action of internal and external stressors and maintain the deformability necessary to carry out their complex functions.Citation1,Citation2
The maintenance of the fluidity and functionality of a membrane depends on several factors, which include the relative content of phospholipids and cholesterol, the concentration of ionsCitation3 and the osmolarity of the medium.Citation4
In erythrocytes, the membrane fluidity contributes to the deformability required for the passage of these cells through small diameter capillaries as well as for the exchange of gases between hemoglobin (Hb) and the external tissues. However, during their lifetime, the red cells undergo various chemical and mechanical stresses, resulting in loss of membrane area and deformability.Citation5,Citation6
The behavior of erythrocyte membrane is influenced by the supply conditions of the lipid constituents of the membrane,Citation7 different kinds of erythrocytopathies and other pathological conditions,Citation3 gender, aging,Citation8 and many chemical agents.Citation9
The in vitro evaluation of the stability of the erythrocyte membrane in relation to hypotonic stress and the chaotropic action of solutes such as ethanol and sodium dodecyl sulfate (SDS)Citation8,Citation10–Citation15 is an essential task to investigate the myriad of conditions that affect the structural homeostasis of biological membranes.
These conditions surely comprise the concentration of many blood biochemical and some hematological variables, which also depend on the nutritional and health status and the use of many types of drugs.
It is within this context that this study evaluates the influence of hematological and biochemical variables on the stability of human erythrocyte membrane.
Methods
Population
The research was approved by the Ethics Committee of the Federal University of Uberlândia (Protocol 127/11). The sample population consisted of 105 female volunteers in the age range of 20–90 years (11 (19–25), 24 (25–31), 5 (31–37), 7 (37–43), 5 (43–49), 5 (49–55), 8 (55–61), 14 (61–67), 8 (67–73), 11 (73–79), 3 (79–85), 4 (85–91)). Only women were recruited to avoid possible physiological variations caused by gender.Citation16,Citation17 The participants of this study were recruited in a clinical laboratory (Labormed, Uberlândia, MG, Brazil) and selected after interview conducted before donation of blood samples. All subjects selected were non-smokers and non-chronic consumers of alcohol. We excluded subjects with known clinical history of chronic diseases (diabetes, coronary heart disease, and dementia) and those who were under chronic drug use.
Blood collection
After overnight (8–12 hours) fasting, blood was collected by intravenous puncture into evacuated tubes (4 ml) with (1) 1.8 mg/ml K3EDTA, for collection of the whole blood, (2) 1.8 mg/ml K3EDTA and 3 mg/ml NaF for determination of glucose, and (3) a spray-dried clot activator, for serum separation.
Determination of the stability of human erythrocytes in relation to sodium dodecyl sulfate
Test tubes with 1 ml of 68–176 µM SDS solutions in 0.9 g dL−1 NaCl were pre-incubated at 37°C for 10 minutes. After addition of 10 µl of whole blood, gently mixing, and incubation for 30 minutes at 37°C, the tubes were centrifuged at 1500 × g for 10 minutes. The supernatants were used for evaluation of the optical density at 540 nm (A540) in an UV-VIS spectrophotometer (Shimadzu™, model UV1650TC, Japan). The graphics of A540 in relation to the SDS concentration were fitted to sigmoidal regression lines, according to the Boltzmann equation:
1
where A1 and A2 represent the minimum and maximum plateaus of A540, D is the SDS concentration, D50 is the SDS concentration capable of promoting 50% of hemolysis, and dD represents the variation in the SDS concentration responsible for the transition of hemolysis.
Determination of hematological and biochemical variables
Hemogram was obtained using an automated system of analysis (Sysmex K4500; Sysmex Corporation™, Mundelein, IL, USA). The lipid profile was performed in an automatic analyzer (Hitachi 917, Roche Diagnostics™, Indianapolis, IN, USA). Serum albumin was colorimetrically assayed using a commercial kit (Labtest, Belo Horizonte, MG, Brazil) and an UV-VIS spectrophotometer (Shimadzu™, model UV1650TC, Japan).
The reference values (women over 16 years of age) were erythrocytes (RBC), 3.9–5.9 millions mm3; Hb, 12.0–16.0 g dl−1; hematocrit, 35.6–48.6%; mean corpuscular volume (MCV), 82–98 fl; mean corpuscular hemoglobin (MCH), 27–31 pg; mean corpuscular hemoglobin concentration (MCHC), 32.9–36%; red cell distribution width (RDW), 12–15%; glucose (Glu), 60–99 mg dl−1; albumin (HSA), 3.5–5.2 g dl−1; total cholesterol (t-C), <200 (optimum) and ≥240 mg dl−1 (high); high-density lipoprotein cholesterol (HDL-C), <45 (low) and >65 mg dl−1 (ideal), low-density lipoprotein cholesterol (LDL-C), <100 (good) and >160 mg dl−1 (high); very low density lipoprotein (VLDL-C), up to 40 mg dl−1; and triglycerides (TG), <150 (optimum) and >201 mg dl−1 (high).
Statistical analysis of experimental data
Data were tested for normality using the D'Agostino-Pearson test.
In an initial analysis, the study population was stratified by age into two groups (20–50 and 51–90 years), using a cutoff age at which the average population of the region of study enters menopause.Citation18 The two groups were compared with respect to all the variables considered in the study, using Student's t-test for normally distributed data and Mann–Whitney test for non-normally distributed data.
The study also used bivariate and multivariate statistics to search for the existence of relations between the erythrocyte stability parameters and the hematological and biochemical variables.
The existence of bivariate linear correlations between the stability parameters (D50 and dD) and the hematological and biochemical variables used the significance level of 0.05. These statistical analyses were performed with the use of the software package OriginPro 9 (MicroCal™, Northampton, MA, USA).
The multivariate analyses were performed by multiple linear regression (MLR), with the help of the software BioEstat 5.0 (Mamirauá™, Belém, PA, Brazil). In the MLR analyses, D50 or dD were considered the dependent variable and the hematological or biochemical parameters constituted the groups of independent variables. In this kind of analysis, the partial regression coefficient measures the strength of the relation between the dependent variable (D50 or dD) and a single independent variable of the group (hematological or biochemical), while the determination coefficient (RCitation2) measures the proportion of variance of the dependent variable (around its mean) that is explained by the whole set of independent variables.
Results
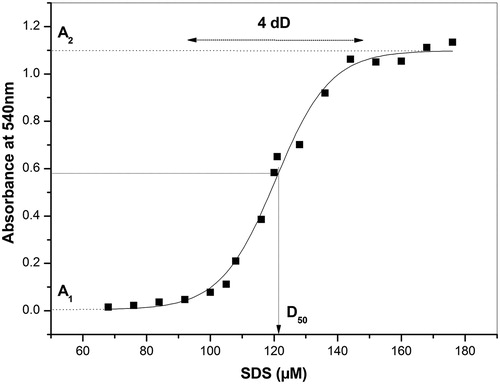
shows a typical curve of erythrocytes lysis induced by increasing concentrations of SDS. Non-linear regression analysis (sigmoidal fitting) was always used to provide the parameters D50, dD, A1 and A2, also showed in the figure.
Figure 1. Sigmoidal fitting of a typical curve of hemolysis promoted by SDS. D50 is the concentration of SDS capable of promoting 50% of hemolysis. dD represents the change in SDS concentration responsible for the hemolysis transition. A1 and A2 represent the minimum and maximum average values of absorbance, respectively.
The preliminary analysis of the results obtained for the entire study population using the D'Agostino-Pearson test has shown the occurrence of normal distribution in the data of all the parameters considered in this study. But after stratification of the population by age, some results did not show normal distribution ().
Table 1. Comparison of stability, biochemical and hematological variables between age ranges
The comparison of the studied variables between the two age groups (20–50 and 51–90 years) showed that the mean values of the stability variable D50, the biochemical variables Glu, VLDL-C, LDL-C, t-C, and TG, and the hematological variables MCV and RDW were significantly higher in the 51–90 years group in relation to the other group. But the average of the MCHC values was significantly lower in 51–90 years group.
shows the matrix of all the possible bivariate correlations between stability parameters (D50 and dD), age and hematological and biochemical variables. Within the limits of statistical significance considered in this study (P < 0.05), age was positively correlated with the values of D50, MCV, RDW, Glu, TG, t-C, and VLDL-C, but negatively associated with the values of MCHC. Regarding the variables of stability, D50 was positively correlated with dD, age, Alb, t-C, LDL-C and HDL-C, while dD was positively correlated with MCH, MCHC, TG, t-C, LDL-C and HDL-C, but negatively correlated with Amin.
Table 2. Matrix of correlations (r) between pairs of biochemical, hematological, and membrane stability variables
The influence of hematological and biochemical variables on the stability parameters (D50 and dD) was analyzed by MLR (). The power of MLR was 80% for an alpha error of 5% (P < 0.05) and an expected variance in the values of the stability variables of 15%. Both the set of biochemical variables and the set of hematological variables have been significantly correlated with the stability variable dD, but the stability variable D50 was significantly correlated only with the set of biochemical variables. The dependent variable dD was significantly correlated with the hematological variables RDW and MCHC and the biochemical variables TG, LDL-C, and HDL-C. The dependent variable D50 was significantly correlated with the biochemical variables Alb and HDL-C, as well as with the age of the participants.
Table 3. Multiple linear regression for D50 and dD in relation to groups of independent variables
Discussion
Although SDS, as detergent, is capable of solubilizing lipid components of biological membranes, at lower concentrations and under the experimental conditions of this study (30-minute incubation time) it has no major impact on the stability of the membrane. The plateau in the absorbance of free Hb designated as A1 in is a proof of this statement. However, a progressive increase in the concentration of this detergent is associated with an (exponential) rise in the release of Hb up to a (SDS) concentration that defines the midpoint of the hemolysis curve (D50), beyond which an increase in its concentration is associated with a hyperbolic rise in the Hb release (). That is why both D50 and dD are constants that present direct dependencies with the chemical stability of the erythrocyte membrane. The hemolysis by SDS is a complex process that can involve intercalation of this detergent in the cell membrane, leading to water penetration and membrane rupture (osmotic mechanism), but it is believed that hemolysis occurs by a process which is essentially based in the solubilization of lipid constituents of the cell membrane (chemical mechanism).Citation14,Citation15,Citation19,Citation20
This study found a positive correlation between D50 and age (), indicating that the older female volunteers have erythrocytes that are more stable against the hemolytic action of SDS. Indeed, the group of older volunteers (51–90 years) presented higher values of the stability variable D50 but not dD in relation to the younger participants (20–50 years) (). Since the stratification by age of these groups was based on the presumed age of entry in menopause, it is possible that hormonal and/or menstrual changes are associated with the erythrocyte stabilization with aging. The fact that menopause is a landmark in the transition of behavior of the erythrocytes was presented by Raval et al.,Citation17 which reported that red cells of stored blood samples from postmenopausal women showed higher mechanical fragility (or lower mechanical resistance) than those from premenopausal women. This study agree with the existence of this landmark, although mechanical resistance and chemical stability are conceptually different properties that cannot be compared in a single basis. Moreover, it is well known that blood storage modifies the structure of the erythrocytes,Citation3,Citation21 which may no longer represent the clinical conditions of their donors. That is why this study used only freshly collected blood samples to perform all tests.
Anyhow, an increased osmotic stability had already been associated to aging in female volunteers aged 20–90 years.Citation8 The causes of such stabilizing effects must involve hematological and biochemical factors that may be better understood by examining the results of this study.
Concerning the hematological variables, the bivariate correlations presented in show that aging was associated with increased MCV and RDW and decreased MCHC in the studied population.
Multivariate statistical analysis was conducted in a tentative to clarify the relationships between the hematological changes associated with aging and changes in the variables of stability. The group set out to explain the variable D50 presented higher predictive ability (RCitation2 adj = 0.3453) and higher multiple correlation coefficient (0.6138) than the group set out to explain the variable dD ().
Although the stability variable that was related to age had been D50 and not dD, it is important to understand the inter-relations of this parameter with the hematological variables considered in this study. The variable dD exhibited positive bivariate correlations with MCH and MCHC (). The MLR showed that RDW was the predictor hematological variable that most strongly correlated with the stability variable dD ().
The MCV is a measure of the average volume of erythrocytes and increases with nutritional deficiencies of folate and/or cobalamin, which are very common among older individuals.Citation22 The RDW is a measure of the variability of erythrocyte volume and their values increase with increasing heterogeneity in the volume of these cells.Citation23
Recent studies have shown that high RDW is strongly associated with increased risk of death and cardiovascular diseaseCitation24–Citation26 in older adults.Citation27 But the mechanisms underlying the increase in RDW with increasing age and associated with mortality are not yet well defined. The positive correlation of the stability variable dD with RDW suggests that the mechanism by which that hematological variable is exerting its influence on the health prognosis of the patient is an excessive elevation in the membrane stability of RBC.
The stability variable D50 showed positive bivariate correlations with t-C, LDL-C, HDL-C and also Alb (). In MLR, albumin appeared as the biochemical variable with the strongest and significant association with D50 (). Certainly, the protective effect of albumin on the stability of erythrocyte membrane in relation to SDS is due to the capacity of this protein of binding to this denaturant,Citation28,Citation29 reducing its hemolytic action.Citation13
On the other hand, the stability variable dD showed significant bivariate and multivariate correlations with TG, LDL-C, and HDL-C (Tables and ).
Besides the hematological causes of the stability increase, there are causes associated to the offer conditions of membrane constituents, such as cholesterol. High levels of cholesterol and triglycerides are common in older adults;Citation30 indeed, these trends were observed in the comparison between the age groups 20–50 and 51–90 years old (). The inclusion of free cholesterol into a biological membrane is an important mechanism of control of its fluidity. As shown in the classic studies done by Cooper,Citation31–Citation34 cholesterol present in the LDL can diffuse into the membrane of blood cells, contributing to regulation of its fluidity. The membranes of these blood cells, consisting predominantly of erythrocytes, can receive the excess of cholesterol from the blood and this, of course, has implications on its physicochemical properties. To the extent that the insertion of cholesterol contributes to membrane reach its critical fluidity, this process leads to the stabilization of these cells and certainly to increase in their time of permanence in blood, with consequent increase in blood populations of RBC. These statements can be supported by the positive correlation observed between the levels of total and LDL-C and the stability of erythrocyte membrane in a population of obese patients submitted to bariatric surgery.Citation35
In fact, patients with familial hypercholesterolemia presented increased content of cholesterol in the erythrocyte membraneCitation36 and mammalians erythrocytes showed decreased osmotic fragility with increase of membrane cholesterol.Citation37 Furthermore, a decrease in the blood levels of cholesterol is associated to a lower ratio between cholesterol and phospholipids in the membrane, with physicochemical changes as increased membrane fluidity.Citation38 These reports from the literature are consistent with the correlations found in this study between the stability variables and the blood concentrations of LDL-C in the bivariate and multivariate correlation analyses.
On the other hand, in experimental animals with considerably higher levels of cholesterol, the excessive diffusion of cholesterol to the cell membrane will manifest as hemolytic anemia.Citation33,Citation39
This means that there must be a threshold of non-HDL-cholesterol associated with the inversion in the dependence of the stability of erythrocyte membrane and non-HDL-cholesterol levels, since the levels of non-HDL-C are correlated to the membrane properties.Citation40
As the increase in RDW, recently characterized as an important prognostic biomarker of cardiovascular diseaseCitation26,Citation41–Citation44 was associated with an increase in cholesterol content of membrane,Citation26 it is quite possible that the predictive ability of RDW is associated with changes in the stability and function of the erythrocyte membrane. Indeed, the negative bivariate correlation of RDW with HDL-C and the positive bivariate correlations of RDW with VLDL-C and TG (), observed in this study, agrees with the association between high RDW values and the etiopathology of the cardiovascular disease.
Conclusions
The values of D50 but not dD enhanced with increasing age of the volunteers in this study, which means that the erythrocyte stability in relation to SDS increased with the volunteers’ age. Analyses of MLR showed that the blood levels of albumin are the main factor that influences the stability variable D50 and that the stability variable dD is associated with increased values of RDW.
Acknowledgements
We would like to thank FAPEMIG (CDS-APQ-01862-09, CDS-APQ-02025-10 and PPM-00485-12), CAPES (PE-PNPD AUX 2718/2011) and CNPq (307705/2012-9) for the financial supports that have enabled this study.
References
- Nash GB, Meiselman HJ. Red cell and ghost viscoelasticity. Effects of hemoglobin concentration and in vivo aging. Biophys J. 1983;43(1):63–73.
- Starodubtseva MN. Mechanical properties of cells and ageing. Ageing Res Rev. 2011;10(1):16–25.
- Girasole M, Pompeo G, Cricenti A, Longo G, Boumis G, Bellelli A, et al. The how, when, and why of the aging signals appearing on the human erythrocyte membrane: an atomic force microscopy study of surface roughness. Nanomedicine 2010;6(6):760–8.
- Lemos GS, Marquez-Bernardes LF, Arvelos LR, Paraiso LF, Penha-Silva N. Influence of glucose concentration on the membrane stability of human erythrocytes. Cell Biochem Biophys. 2011;61(3):531–7.
- Waugh RE, Narla M, Jackson CW, Mueller TJ, Suzuki T, Dale GL. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood 1992;79(5):1351–8.
- Bransky A, Korin N, Nemirovski Y, Dinnar U. Correlation between erythrocytes deformability and size: a study using a microchannel based cell analyzer. Microvasc Res. 2007;73(1):7–13.
- de Freitas MV, de Oliveira MR, dos Santos DF, de Cassia Mascarenhas Netto R, Fenelon SB, Penha-Silva N. Influence of the use of statin on the stability of erythrocyte membranes in multiple sclerosis. J Membr Biol. 2010;233(1–3):127–34.
- Penha-Silva N, Firmino CB, de Freitas Reis FG, da Costa Huss JC, de Souza TM, de Freitas MV, et al. Influence of age on the stability of human erythrocyte membranes. Mech Ageing Dev. 2007;128(7–8):444–9.
- Penha-Silva N, Arvelos LR, Cunha CC, Aversi-Ferreira TA, Gouvea-e-Silva LF, Garrote-Filho MS, et al. Effects of glycerol and sorbitol on the thermal dependence of the lysis of human erythrocytes by ethanol. Bioelectrochemistry 2008;73(1):23–9.
- Bernardino Neto M, de Avelar EB, Jr., Arantes TS, Jordão IA, da Costa Huss JC, de Souza TMT, et al. Bivariate and multivariate analyses of the correlations between stability of the erythrocyte membrane, serum lipids and hematological variables. Biorheology 2013;50(5–6):305–20.
- Cunha CC, Arvelos LR, Costa JO, Penha-Silva N. Effects of glycerol on the thermal dependence of the stability of human erythrocytes. J Bioenerget Biomembranes 2007;39(4):341–7.
- de Freitas MV, Netto Rde C, da Costa Huss JC, de Souza TM, Costa JO, Firmino CB, et al. Influence of aqueous crude extracts of medicinal plants on the osmotic stability of human erythrocytes. Toxicol In Vitro 2008;22(1):219–24.
- Fonseca LC, Arvelos LR, Netto RC, Lins AB, Garrote-Filho MS, Penha-Silva N. Influence of the albumin concentration and temperature on the lysis of human erythrocytes by sodium dodecyl sulfate. J Bioenerg Biomembr. 2010;42(5):413–8.
- Shalel S, Streichman S, Marmur A. The mechanism of hemolysis by surfactants: effect of solution composition. J Colloid Interface Sci. 2002;252(1):66–76.
- Shalel S, Streichman S, Marmur A. Modeling surfactant-induced hemolysis by Weibull survival analysis. Colloids Surfaces B-Biointerfaces 2003;27(2–3):223–9.
- Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, Daly AR, et al. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010;99(4):325–31.
- Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, Kameneva MV, et al. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011;100(4):418–21.
- Pedro AO, Pinto Neto AM, Paiva LH, Osis MJ, Hardy E. Age at natural menopause among Brazilian women: results from a population-based survey. Cad Saude Publica 2003;19(1):17–25.
- Bielawski J. Two types of haemolytic activity of detergents. Biochim Biophys Acta 1990;1035(2):214–7.
- Hooghwinkel GJ, de Rooij RE, Dankmeijer HR. The mechanism of hemolysis by various types of surfactants. Acta Physiol Pharmacol Neerl 1965;13(3):304–16.
- Pompeo G, Girasole M, Cricenti A, Boumis G, Bellelli A, Amiconi S. Erythrocyte death in vitro induced by starvation in the absence of Ca(2+). Biochim Biophys Acta 2010;1798(6):1047–55.
- Alves de Rezende CH, Coelho LM, Oliveira LM, Penha Silva N. Dependence of the geriatric depression scores on age, nutritional status, and haematologic variables in elderly institutionalized patients. J Nutr Health Aging 2009;13(7):617–21.
- Batool S, Wang Q, Qureshi S, Chua E. The red cell diameter width distribution, the forgotten haematological parameter for anaemia in the older person. Eur Geriatr Med. 2013;4:1–4.
- Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50(1):40–7.
- Cavusoglu E, Chopra V, Gupta A, Battala VR, Poludasu S, Eng C, et al. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol. 2010;141(2):141–6.
- Tziakas D, Chalikias G, Grapsa A, Gioka T, Tentes I, Konstantinides S. Red blood cell distribution width: a strong prognostic marker in cardiovascular disease: is associated with cholesterol content of erythrocyte membrane. Clin Hemorheol Microcirc. 2012;51(4):243–54.
- Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65(3):258–65.
- Pitt-Rivers R, Impiombato FS. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968;109(5):825–30.
- Shweitzer B, Zanette D, Itri R. Bovine serum albumin (BSA) plays a role in the size of SDS micelle-like aggregates at the saturation binding: the ionic strength effect. J Colloid Interface Sci. 2004;277(2):285–91.
- Hausman DB, Fischer JG, Johnson MA. Protein, lipid, and hematological biomarkers in centenarians: definitions, interpretation and relationships with health. Maturitas 2012;71(3):205–12.
- Cooper RA, Durocher JR, Leslie MH. Decreased fluidity of red cell membrane lipids in abetalipoproteinemia. J Clin Invest. 1977;60(1):115–21.
- Cooper RA, Leslie MH, Fischkoff S, Shinitzky M, Shattil SJ. Factors influencing the lipid composition and fluidity of red cell membranes in vitro: production of red cells possessing more than two cholesterols per phospholipid. Biochemistry 1978;17(2):327–31.
- Cooper RA, Leslie MH, Knight D, Detweiler DK. Red cell cholesterol enrichment and spur cell anemia in dogs fed a cholesterol-enriched atherogenic diet. J Lipid Res. 1980;21(8):1082–9.
- Cooper RA. Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med. 1977;297(7):371–7.
- de Arvelos LR, Rocha VC, Felix GP, da Cunha CC, Bernardino Neto M, da Silva Garrote Filho M, et al. Bivariate and multivariate analyses of the influence of blood variables of patients submitted to Roux-en-Y gastric bypass on the stability of erythrocyte membrane against the chaotropic action of ethanol. J Membr Biol. 2013;246(3):231–42.
- Michalska-Malecka K, Slowinska L, Dorecka M, Romaniuk W. Correlations in some pathogenetic factors and values of hemorheological parameters in age-related macular degeneration. Clin Hemorheol Microcirc. 2008;38(3):209–16.
- Coldman MF, Gent M, Good W. Relationships between osmotic fragility and other species-specific variables of mammalian erythrocytes. Comp Biochem Physiol. 1970;34(4):759–72.
- Cazzola R, Rondanelli M, Trotti R, Cestaro B. Effects of weight loss on erythrocyte membrane composition and fluidity in overweight and moderately obese women. J Nutr Biochem. 2011;22(4):388–92.
- Akahane K, Furuhama K, Onodera T. Simultaneous occurrence of hypercholesterolemia and hemolytic anemia in rats fed cholesterol diet. Life Sci. 1986;39(6):499–505.
- Rabini RA, Polenta M, Staffolani R, Tocchini M, Signore R, Testa I, et al. Effect of hydroxymethylglutaryl-CoA reductase inhibitors on the functional properties of erythrocyte membranes. Exp Mol Pathol. 1993;59(1):51–7.
- Cauthen CA, Tong W, Jain A, Tang WH. Progressive rise in red cell distribution width is associated with disease progression in ambulatory patients with chronic heart failure. J Card Fail. 2012;18(2):146–52.
- Nishizaki Y, Yamagami S, Suzuki H, Joki Y, Takahashi S, Sesoko M, et al. Red blood cell distribution width as an effective tool for detecting fatal heart failure in super-elderly patients. Intern Med. 2012;51(17):2271–6.
- Tziakas DN, Chalikias GK, Stakos D, Boudoulas H. The role of red blood cells in the progression and instability of atherosclerotic plaque. Int J Cardiol. 2010;142(1):2–7.
- Michel JB, Virmani R, Arbustini E, Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J. 2011;32(16):1977–85 , 1985a, 1985b, 1985c.
