Abstract
Background
Bacterial contamination of platelet products is the major infectious risk in blood transfusion medicine, which can result in life-threatening sepsis in recipient. Lipocalin 2 (Lcn2) is an iron-sequestering protein in the antibacterial innate immune response, which inhibit bacterial growth. This study was aimed to evaluate the antibacterial property of Lcn2 in preventing bacterial contamination of platelets.
Methods
Recombinant Lcn2 was expressed in a eukaryotic expression system and following purification and characterization of the recombinant Lcn2, its minimum inhibitory concentration was determined. Then, platelet concentrates were inoculated with various concentrations of Staphylococcus epidermidis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, and Enterococcus faecalis, and the antibacterial effects of Lcn2 was evaluated at 20–24°C.
Results
Results revealed that Lcn2 effectively inhibited the growth of 1.5 × 104 CFU/ml S. epidermidis, P. aeruginosa, K. pneumoniae, E. coli, and E. faecalis at 40 ng/ml. At this concentration, Lcn2 also inhibited the growth of 1.5 × 103 CFU/ml Staphylococcus aureus and Proteus mirabilis.
Conclusion
Recombinant Lcn2 inhibited growth of a variety of platelet-contaminating bacteria. Therefore, supplementation of platelet concentrates with Lcn2 may reduce bacterial contamination.
Introduction
In comparison to red blood cells, platelets cannot tolerate cold storage (4°C), and if refrigerated, are quickly cleared from blood circulation following transfusion.Citation1,Citation2 Thus, platelets are stored at room temperature (20–24°C) under constant agitation. This makes favorable conditions for bacterial growth.Citation3 Hence, platelet preparations are more susceptible to contamination than the other blood products.Citation4
It is estimated that the rate of bacterial contamination of platelet units is about 1 in 2000–3000 units (in whole-blood and apheresis-derived platelets).Citation3,Citation5 Bacterial infections are the main causes of acute morbidity and mortality among post-transfusion infections.
Sources of bacterial contamination include contamination during whole-blood collection procedure, donor bacteraemia, contamination of collection packs, or contamination during blood processing procedure. Contamination at the time of blood collection is the major cause of bacterial contamination and most organisms are originated from skin normal flora.Citation4,Citation6 It may be virtually impossible to completely decontaminate human skin, therefore, to prevent or limit bacterial contamination, additional precautions such as discarding the first 20–30 ml of donated blood must be considered.
Several methods have been investigated for detection of bacteria in platelet products including culture based methods (pall eBDS and BacT/ALERT systems), measurement of pH, and glucose concentration at time of issue etc. Cultivation is currently the mostly used method to detect bacteria; however, it is time consuming and platelets may be transfused before the BacT/ALERT become positive. Also, slow growing bacteria, or low bacterial load might be missed.Citation7
Low amounts of bacteria at collection time (<10 CFU/ml) can change into very high amounts (>1 × 108 CFU/ml) during storage.Citation8 This problem can potentially be prevented using a substance that inactivates bacteria or that inhibits bacterial growth during storage. Since platelet products should be transfused, it is not reasonable to use antibiotics to inhibit platelets contamination. Therefore, an antibacterial agent naturally present in human tissues might be considered promising.
Lipocalin 2 is a secretory protein which initially isolated from neutrophils. The neutrophil gelatinase-associated lipocalin (NGAL), or lipocalin-2/24p3, belongs to the super family of structurally related small extracellular lipocalin proteins with great functional diversity.Citation9–Citation11 It is thought to be an acute phase protein whose expression is induced under harmful conditions such as intoxication, renal injury, burn injury, human cancers, inflammatory bowel disease, infection, and other forms of cellular stresses.Citation12–Citation18 However, one of the well-known functions of Lcn2 is inhibition of microorganism's growth. The mechanisms underlying Lcn2 antibacterial effects is not fully understood, however, interfering with iron acquisition via siderophore is the most acceptable mechanism now.Citation19,Citation20 In this study, recombinant lipocalin 2 has been used as a potential protective factor against bacterial contamination of platelets.
Methods and material
Cell culture
HepG2 (human hepatoma cell line) and HEK293T (human embryonic kidney cell line) cells were obtained from National Cell Bank of Iran (NCBI, Pasteur Institute, Iran). The cells were grown in RPMI-1640 medium (Gibco-BRL, Eggenstein, Germany) supplemented with 10% fetal bovine serum (Gibco-BRL), 100 U penicillin/ml and 100 mg/ml streptomycin.
Preparation of recombinant Lcn2
pCDNA3.1(+) (Invitrogen, Carlsbad, CA,USA) plasmid was used for cloning and expression of recombinant Lcn2 by the HEK293T cells. In this regard, total RNA was extracted from HepG2 cells by Tripure reagent (Invitrogen) according to the manufacturer's protocol. Then, reverse transcription was performed by SuperScript III reverse transcriptase (Invitrogen) with 500 ng of the extracted total RNA as described elsewhere,Citation21 followed by amplification of full-length human Lcn2 through reverse transcription polymerase chain reaction (RT-PCR) and its subsequent cloning in the pCDNA3.1(+) plasmid. The cloning procedure was performed in DH5α strain of E. coli and the fidelity of cloning was evaluated by DNA sequencing. The recombinant plasmid was designated as pCDNA-lcn2 and transfected to the HEK293T cells and the cells were cultivated in serum free medium (PEM; Invitrogen). Following expression of the recombinant Lcn2, the culture medium was filtered through miniprep (ultra filtrations) followed by purification of Lcn2 with DEAE sepharose CL-6B columns (Sigma, Germany). Then the purified protein was dialyzed against gradient solution of NaCl. Citation21,Citation22The purified Lcn2 was made endotoxin-free using Detoxi-Gel endotoxin removing column (Thermo Scientific, Pittsburgh, PA, USA) as recommended by the manufacturer. The protein was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), western blotting, and enzyme-linked immunosorbent assay (ELISA). Protein concentration was determined by Bradford assay (Bio-Rad, CA, USA) and using human Lcn2 ELISA kit (R& D, USA).
Western blot
Western blot analysis was performed to authenticate the purified protein. In this regard, protein bands were separated using 12% SDS-PAGE and then transblotted to PVDF membrane (Roche, Germany). Afterwards, the membrane was incubated with blocking buffer containing 5% BSA for 1 hour at 4°C followed by its washing with TBS containing 0.1% Tween 20 (wash buffer), and incubation with anti human Lcn2 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Following an overnight incubation at 4°C with the primary antibody, the membrane was washed with the wash buffer, and incubated with secondary HRP conjugated anti mouse antibody (Santa Cruz, CA). Finally, the membrane was developed with DAB solution (Sigma).
Bacterial strains and culture conditions
The E. coli strain HB101 (ATCC 33694), Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228), Pseudomonas aeruginosa (ATCC 27853), Klebsiella pneumoniae (ATCC 1053), Enterococcus faecalis (ATCC 29212), and Proteus mirabilis (ATCC 15146) were used in this study. The bacteria were grown overnight in Luria-Bertani (LB) broth while shaking at 37°C before being used for the experiment. Then, the bacteria were cultured on blood agar medium to obtain isolated colonies. Following overnight incubation at 37°C, well-isolated colonies were transferred to a tube containing sterile saline and vortexed thoroughly.
Preparation of bacterial inoculums for determination of minimum inhibitory concentration
The density of bacterial inoculums was standardized with the 0.5 McFarland turbidity standard. Bacterial suspensions were prepared by transferring a fresh colony to sterile saline and subsequent mixing. Then, the suspension turbidity was compared with turbidity of 0.5 McFarland standard by holding in front of a light against a white background, with contrasting black lines.Citation23
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) was calculated with Broth macro dilution method.Citation23 In this regard, sets of glass tubes containing 1 ml Mueller Hinton Broth (MHB) were inoculated with 1 ml of 1.5 × 108 CFU/ml of the bacterial suspensions. Then, the recombinant Lcn2 was added to the tubes at final concentrations of 50, 25, 12.5, 6.25, 3.12, and 1.56 ng/ml, followed by their incubation at 22° C for 24 hour, and finally, the MIC was evaluated. Positive and negative controls of the test included some tubes containing bacteria but not Lcn2, and some tubes without both Lcn2 and bacteria, respectively. All experiments were carried out in triplicate.
Assessment of Lcn2 antibacterial effects in platelet concentrates
For this experiment, bags of human PLT concentrates (PCs) separated from whole blood were obtained from Iranian Blood Transfusion Organization (IBTO, Iran). In this regard, after written informed consent, whole blood was collected from healthy human volunteers who had not taken any medicine during the preceding 3 weeks, and mixed with CPDA-1 (citrate phosphate dextrose adenine. After viral screening, the donated whole bloods were pooled (to eliminate inter-bag variation) and centrifuged with light spin (2000 g) for 4 minutes at room temperature (20–24°C) to separate red cells from plasma. The platelet-rich plasma (PRP) is then centrifuged at room temperature with hard spin (4000 g) for 9 minutes to concentrate the platelets. Five milliliters of the platelet concentrate was added to 15 ml falcons under sterile condition, followed by addition of Lcn2 (100 ng/ml) and 1 ml of various titer (1.5 × 107–1.5 × 103 CFU/ml) of the bacteria. Final concentration of Lcn2 was 40 ng/ml and the bacterial loads were between 1.5 × 106–1–5 × 102 CFU/ml. The mixtures were incubated at 22 ± 2°C while shaking for 4 days, and then the samples were cultivated on blood agar plates and inspected for bacterial growth. For control of experiment conditions, positive (without Lcn2) and negative (without Lcn2 and bacteria) controls were controls were used. Since the positive controls did not contain Lcn2, it was expected that the bacteria grew up freely. In case of negative controls, in which only platelet concentrates were added to the culture media, no bacterial growth must be observed. This was used to check potential contamination of the tubes during their incubation in room temperature for four days. All experiments were carried out in triplicate. Then, 100 µl of the above mixtures were cultured on Luria-Bertani (LB) agar plates and results were compared with those of the controls.
Quality of PCs
According to the protocol that mentioned above, PRP was separated from whole blood by light centrifugation and under this condition, platelets remained suspended in plasma. Lcn2 (40 ng/ml) was added to PRP and bags were incubated at 22 ± 2°C while shaking for 4 days. Then, the bags contents were assayed for platelet count, pH, and aggregation (on days 1, 2, and 4), and compared with control PRP (without Lcn2). The PLT number was determined with a blood cell counter (Sysmex K-1000). pH was measured with pH meter (Metrohm, Switzerland). PLT aggregation assayed in response to 10 µmol/l adenosine 5′-diphosphate, 400 µg/ml arachidonic acid, and 0.8 mg/ml ristocetin with aggregometer (Chrono-log).
Results
Expression and purification of recombinant Lcn2
Lcn2 cDNA was isolated from HepG2 cell line and cloned into the pcDNA3.1 vector. Then, the HEK293T cells were stably transfected with the recombinant pCDNA-Lcn2 or empty vectors (). Expression of Lcn2 mRNA and protein by the recombinant HEK293T cells were assayed with RT-PCR and ELISA (A and ). In addition, Lcn2 protein was further purified and confirmed by Western blotting (B). The presence of a band of about 27.7 kDa confirmed the purity of recombinant Lcn2.
Figure 1. Summary of construction of recombinant vector and its transfection and purification. HepG2 cell line was used as a source for Lcn2 cDNAs. The isolated gene was cloned into pcDNA3.1 vector. Stable clones expressing recombinant Lcn2 was established in the presence of geneticin. Finally, Lcn2 was purified.
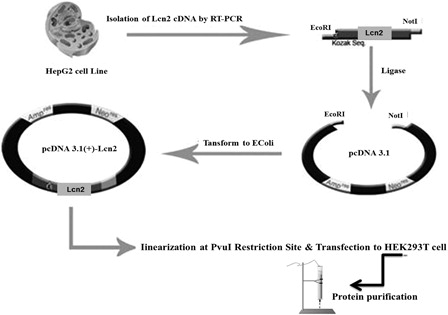
Figure 2. Expression of recombinant Lcn2 by HEK293T cells: (A) RT-PCR: RT-PCR analysis showed high levels of Lcn2 expression by the stable HEK293T cells. No detectable amount of expression was observed in non-transfected HEK293T. The HEK293T-Lcn2 showed a 642-bp fragment (lane V-Lcn2), whereas no expression was detected in HEK293T transfected with the non-recombinant pcDNA3.1 plasmid (HEK293T-V) (lane V). M, 100 bp DNA marker. (lower image) Expression of β-actin was used for normalization. M, 100 bp DNA marker. (B) Western blot analysis of Lcn2 after purification. Control HEK293T-V (lane V) revealed no detectable expression of Lcn2 protein compared to the HEK293T-Lcn2 (lane V-Lcn2).
Table 1. Results of the purified human Lcn2 immunoassay (ELISA)
Determination of minimum inhibitory concentration of Lcn2
MIC is defined as the lowest concentration of an antimicrobial agent which will inhibit the visible growth of a microorganism after overnight incubation.Citation23 The MIC of Lcn2 is shown in .
Table 2. Minimum inhibitory concentration of Lcn2*
In this study, the MIC of Lcn 2 was assessed 24 hour after incubation of various concentrations of Lcn2 with a bacterial load of 1.5 × 108 CFU/ml at 22 ± 2°C. Afterwards, platelet concentrates containing 40 ng/ml Lcn2 were inoculated with various titers of the bacterial suspensions, and after four days of incubation at 22 ± 2°C (with shaking), all samples were cultivated on LB-agar plates and inspected for bacterial growth. For each bacterial species, the final loads were 1.5 × 102, 1.5 × 103, 1.5 × 104, 1.5 × 105, and 1.5 × 106 CFU/ml. Results showed that in cases of S. epidermidis, P. aeruginosa, K. pneumoniae, E. coli, and E. faecalis, 40 ng/ml of Lcn2 inhibited the growth of inoculated bacteria in tubes containing platelet concentrates contaminated with 1.5 × 102, 1.5 × 103, and 1.5 × 104 CFU/ml of the bacteria. But, in tubes containing higher bacterial loads, this concentration of Lcn2 did not inhibit bacterial growth. In addition, in case of S. aureus and P. mirabilis, 40 ng/ml of Lcn2 only inhibited the bacterial growth in the tubes containing 1.5 × 102 and 1.5 × 103 CFU/ml bacteria (). It was noteworthy that in case of positive controls (in which platelet samples were inoculated with the bacteria but Lcn2 was not added), all mentioned bacteria grew, indicating that residual white blood cells (WBCs) in non-leukoreduced whole blood platelet units did not inhibit bacterial growth. This showed that the observed bactericidal effect was not due to residual WBCs.
Table 3. Antibacterial effects of Lcn2 in platelet concentrate*
Platelets aggregation in the presence of Lcn2
The effects of Lcn2 on the quality of the PCs are summarized in . The results indicate that recombinant Lcn2 does not affect quality of PCs.
Table 4. The effects of Lcn2 on the quality of the PCs
Discussion
Since platelet products are kept at room temperature, there is always a possibility for bacterial growth. Although, current tools of monitoring the products for bacterial contamination have reduced risk of transfusion of infected platelet preparations, but have not completely removed this problem, and bacterial contamination of platelet products is still a major problem in blood transfusion medicine.
For the first time, in this study we evaluated the bacterioestatic effect of Lcn2 on platelet concentrates contaminated with various bacteria. The antibacterial effect of Lcn2 was previously shown in mice.Citation24 Since Lcn2 has an important role in the innate immunity, Lcn2-deficient mice demonstrated increased sensitivity to E. coli infection. Moreover, it was demonstrated that neutrophils isolated from Lcn2-deficient mice had significantly lower bacteriostatic activity compared with wild-type neutrophils.Citation24
Findings of various studies have indicated that most bacteria associated with transfusion reactions belong to aerobic or facultative species, and anaerobic species are rarely reported to be responsible for the reaction. This is despite frequent isolation of Propionibacterium acnes using aerobic and anaerobic BacT/ALERT culture bottles. Therefore, only aerobic bacteria were selected to be evaluated in this study.
Other studies indicated that Lcn2 is able to inhibit growth of some other bacterial species that do not play role in bacterial contamination of platelet products. For instance, Lcn2 is involved in pulmonary host defense against Klebsiella infection.Citation25 Furthermore, the Mycobacterial growth in alveolar epithelium can be inhibited by Lcn2,Citation26 and expression of Lcn2 is increased in the cells of gastric mucosa infected with Hellicobacter pylori.Citation27 Therefore, Lcn2 is able to inhibit growth of a wide range of bacterial species.
Bacterioestatic mechanism of Lcn2 has been shown to include the interference with iron uptake by bacteria. In a study carried out by Tanaka et al., the possibility of employing a bactericidal polypeptide, ε-poly-l-lysine (εPLL), in platelet concentrates to prevent bacterial contamination was evaluated. The polypeptide ε-poly-l-lysine is a molecule composed of approximately 30 l-lysine subunits, and obtained by purification from a culture fluid produced in aerobic fermentation of a non-pathogenic bacterium, Streptomyces albulus. Considering the antimicrobial activity of the polypeptide, it has been widely used in food industries as a food additive. Tanaka et al. evaluated the antimicrobial effect of εPLL against S. aureus, Bacillus cereus, and Klebsiella oxytoca that were inoculated (20 CFU/ml) into platelet concentrates. According to their results, bacterial growth was inhibited with concentrations between 200 and 50 µg/ml of the polymer after 8 days of incubation.Citation28 This is while our results demonstrated that the inhibitory concentration of Lcn2 is about 40 ng/ml.
However, in order to confirm the effectiveness of supplementing platelet preparations with Lcn2, for prevention of septic reactions upon their application, further confirmatory in vitro and in vivo studies are needed. Furthermore, the safety of Lcn2 supplementation is another concern that must be addressed before its clinical application. So far there is no report dealing with clinical administration of recombinant Lcn2. However, normal circulating level of Lcn2 is about 30–50 ng/mlCitation29,Citation30, somewhat similar to the effective dose defined in the current study (i.e. 40 ng/ml).
Inactivation of pathogens with photochemical compounds such as psoralen or riboflavin has received more attention in recent years. This method leads to elimination of parasites and novel viruses, as well as bacteria. Nevertheless, there are some reports indicating that these methods can reduce quality of the product and decreased the number of platelets after transfusion.Citation8
Overall, in this study we introduced a natural human-derived protein, Lcn2, which acts as an antibacterial agent to inhibit bacterial contamination of platelet concentrates. Since the expression of Lcn2 is up-regulated by innate immunity whenever our body faces bacterial infections, it could be logic to assume that there is no side effect in potential clinical application of Lcn2. Of course, further comprehensive and detailed studies in this regard are required.
References
- Wandall HH, Hoffmeister KM, Sqrensen AL, Rumjantseva V, Clausen H, Hartwing JH, et al. Galactosylation does not prevent the rapid clearance of long-term, 4 0C –stored platelets. Blood 2008;111(6):3249–56.
- Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. TransfusApheresis Sci. 2010;42(1):63–70.
- Vedy D, Robert D, Gasparini D, Canellini G, Waldrogel S, Tissot JD. Bacterial contamination of platelet concentrates: pathogen detection and inactivation methods. Hemtol Rev. 2009;1:e5.
- Palavecino EL, Yomtovian RA, Jacobs MR. Bacterial contamination of platelets. Transfus Apheresis Sci. 2009;42(1):71–82.
- Larsen CP, Ezligini F, Hermansen NO, Kjeldsen-Kragh J. Six years experience of using the BacT/ALERT system to screen all platelet concentrates, and additional testing of outdated platelet concentrates to estimate the frequency of false-negative results. Vox Sang. 2005;88(2):93–7.
- Blajchman MA. Bacterial contamination of cellular blood components: risks, sources and control. Vox Sang. 2004;87(1):98–103.
- Rood IG, Koppelman MH, Pettersson A, Savelkoul PH. Development of an internally controlled PCR assay for broad range detection of bacteria in platelet concentrates. J Microbiol Methods 2008;75(1):64–9.
- Mathi J. Problem of bacterial contamination in platelet in concentrates. Transfus Apheresis Sci. 2009;41:139–44.
- Flower DR. The lipocalin protein family: structure and function. Biochemistry 1996;318(1):1–14.
- Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta (BBA) 2000;1482:272–83.
- Ziegler S, Röhrs S, Tickenbrock L, Langerak A, Chu ST, Feldmann I. Lipocalin 24p3 is regulated by the Wnt pathway independent of regulation by iron. Cancer Genet Cytogen. 2007;174:16–23.
- Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L, et al. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut 1996;38:414–20.
- Seth P, Porter D, Lahti-Domenici J, Geng Y, Richardson A, Polyak K. Cellular and molecular targets of estrogen in normal human breast tissue. Cancer Res. 2002;62:4540–4.
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43.
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004;432:917–21.
- Mishra J, Morib K, Maa Q, Kellya C, Baraschb J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–15.
- Vemula M, Berthiaume F, Jayaraman A, Yarmush ML. Expression profiling analysis of the metabolic and inflammatory changes following burn injury in rats. Physiol Genom. 2004;18:87–98.
- Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391:441–8.
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore mediated iron acquisition. Mol Cell 2002;10(5):1033–43.
- Borregaard N, Cowland JB. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals 2006;19(2):211–5.
- Roudkenar MH, Halabian R, Ghasemipour Z, Roushandeh AM, Rouhbakhsh M, Nekogoftar M, et al. Neutrophil gelatinase-associated lipocalin acts as a protective factor against H(2)O(2) toxicity. Arch Med Res. 2008;39(6):560–6.
- Roudkenar MH, Halabian R, Roushandeh AM, Nourani MR, Masroori N, Ebrahimi M, et al. Lipocalin 2 regulation by thermal stresses: protective role of Lcn2/NGAL against cold and heat stresses. Exp Cell Res. 2009;315(18):3140–51.
- Baron EJ, Finegold SM. Bailey & Scott's diagnostic microbiology. 8th ed. Missouri, United States of America: Amazon; 1990. pp. 171–80.
- Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. Lipocalin 2-deficient mice exhibite increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. PNAS Feb. 2006;103(6):1834–9.
- Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, Berger T, et al. Lipocaline 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009;182(8):4947–56.
- Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, et al. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J. Immunol. 2008;181(12):8521–7.
- Alpízar-Alpízar W, Laerum OD, Illemann M, Ramírez JA, Arias A, Malespín-Bendaña W, et al. Neutrophil gelatinase-associated lipocalin (NGAL/Lcn2) is upregulated in gastric mucosa infected with Hellicobacter pylori. Virchows Arch 2009;455(3):225–33
- Tanaka S, Hayashi T, Tateyama H, Matsumura K, Hyon SH, Hirayama F. Application of the bactericidal activity of e-poly-l-lysin to the storage of human platelet concentrates. Transfusion 2010;50(4):932–40.
- Naudé PJ, Eisel UL, Comijs HC, Groenewold NA, De Deyn PP, Bosker FJ, et al. Neutrophil gelatinase-associated lipocalin: a novel inflammatory marker associated with late-life depression. J. Psychosom Res. 2013;75(5):444–50.
- Roudkenar MH, Halabian R, Oodi A, Roushandeh AM, Yaghmai P, Najar MR, et al. Upregulation of neutrophil gelatinase-associated Lipocalin, NGAL/Lcn2,in b-Thalassemia patients. Arch Med Res. 2008;39(4):402–7.
