Abstract
Background
Sickle cell disease (SCD) is associated with perioperative vascular (SCD-related) and non-vascular complications. To minimize perioperative complications during elective surgery, either exchange blood transfusion or simple blood transfusion can be used. We systematically reviewed the literature and meta-analyzed randomized and observational trials comparing exchange transfusion to simple transfusion, as well as studies comparing preoperative transfusion to no transfusion to assess the relative risk (RR) and benefit of each strategy in sickle cell patients undergoing surgery.
Methods
Medline, Embase, and the Cochrane-controlled trial register were searched to identify studies that evaluated exchange transfusion to simple transfusion, as well as studies comparing any form of blood transfusion with no transfusion. Studies were evaluated according to a priori inclusion criteria and critically appraised using established internal validity criteria. Pooled RR was estimated using a random effects model.
Results
Three randomized trials and seven observational studies were included. We found there was no difference between exchange transfusion and simple transfusion for perioperative mortality, vascular, or non-vascular perioperative complications. However, transfusion-related complications (RR 2.41, 95% confidence interval (CI): 1.49–3.91) and the amount of blood transfused (mean difference 2.03, 95% CI: 1.23–2.83) were higher in those treated with exchange transfusion versus simple transfusion. Similarly, there was no difference in perioperative mortality, vascular, or non-vascular perioperative complications between those treated with preoperative transfusion strategy and no transfusion strategy.
Conclusion
Based on the current literature, neither preoperative exchange transfusion nor simple transfusion reduces perioperative complications in patients with SCD who are undergoing surgery; however, available studies were underpowered to detect a treatment effect.
Introduction
Sickle cell disease (SCD) is a chronic hemolytic anemia that results in multiple vascular complications.Citation1 Despite improvements in the clinical care of SCD patients, the average life expectancy for men and women with homozygous disease (SS) is only 42 years and 48 years, respectively.Citation2 Surgical interventions to treat problems associated with persistent or acute organ dysfunction are relatively common in people with SCD.Citation3 For example, hemolytic anemia causes increased bilirubin levels and a high incidence of gallstones, with subsequent frequent need for cholecystectomy.Citation4 Hypersplenism, splenic sequestration, and infarction may be treated with splenectomy.Citation5 Orthopedic complications resulting from avascular necrosis, bone infection, and musculoskeletal malformations may require joint replacement, drainage, or surgical correction.Citation6
The rate of perioperative complications varies according to the clinical severity of the disorder and the type of operation but, overall, complications in this patients group are common,Citation3,Citation7–Citation9 ranging between 7 and 32% in SCD patients.Citation1 Complications are usually classified as either SCD-related (painful crisis, acute chest syndrome (ACS), and cerebrovascular accident (CVA)), or non-SCD-related (infection, bleeding, thrombosis, and death).Citation9 SCD-related complications are mainly due to secondary hemolysis and decreased microvascular perfusion. Preoperative red blood cell (RBC) transfusion in sickle cell (SC) patients is known to decrease the proportion of hemoglobin S (HbSS), suppress erythropoiesis, and improve anemia, and has been linked to a better postoperative course and decreased perioperative complications in some studies.Citation8,Citation10 Various regimens are used in clinical practice. These include partial or full exchange transfusion or simple transfusion either immediately prior to surgery or up to 14 days prior to surgery to maximize the oxygen transport capacity of the transfused blood.
Currently, there is no consensus over the best method or the necessity of transfusion to reduce perioperative complications in SCD patients undergoing elective surgery. Our objective was to systematically evaluate the available randomized and observational studies to determine the risk and benefits of the different preoperative transfusion strategies.
Methods
Literature search
We systematically searched the published and unpublished medical literature for randomized and observational trials comparing preoperative exchange transfusion to simple transfusion in SCD patients undergoing elective surgery. We also sought studies comparing preoperative transfusion regimen (no restriction on the type of transfusion either simple, exchange, or both) with no transfusion. The Cochrane Central Register of Controlled trials, EMBASE, Medline, and Cumulative Index to Nursing and Allied Health Literature (CINAHIL) databases were searched from inception through April 2013 without limitation on language or study design. The following search terms were used as a Medical Subject Heading terms and/or keywords: sickle or SC anemia, or hemoglobinopathies; transfusion (aggressive or conservative) or preoperative care. To search for unpublished literature, the reviewers searched major hematology societies for abstracts from their annual meetings (American Society of Hematology and European Hematology Society) from 2011 to 2012. Reference list of retrieved studies were hand searched to find studies not identified initially by the electronic search. The detailed information about the type of transfusion and the target Hb and HbSS are shown in .
Table 1. Characteristics of studies included in the quantitative data synthesis
Study selection and data items
Randomized controlled trials (RCTs) and observational studies were included if they fulfilled the following criteria: (i) enrolled patients with SCD undergoing elective surgery; (ii) compared transfusion regimen to a different regimen (i.e. exchange transfusion or simple transfusion) or compared transfusion to no transfusion; (iii) included patients diagnosed preoperatively with SCD (SS), sickle beta thalassemia (Sβ0 and Sβ+), or sickle cell hemoglobin C (SC) proven by hemoglobin electrophoresis and/or sickle solubility test; and (iv) studies report at least one of the following outcomes of interest: (1) Perioperative mortality; (2) Postoperative infection; (3) serious complications related to SCD (painful crisis, ACS, CVA); (4) serious surgical complications, as defined by the author, including a fall in hemoglobin greater than 2 mg/dl; (5) preoperative hemoglobin; (6) preoperative HbSS level %; (7) transfusion-related complications; and (8) volume of transfused packed RBCs (PRBCs). We had no age or sex limitation and included any elective surgical setting. Abstracts were allowed if they included adequate data for quality assessment and analysis. Studies that included SCD patients in emergency setting or used co-intervention in one arm were excluded. Two reviewers independently reviewed titles onlyCitation11 for eligibility in an un-blinded and standardized manner; all disagreements were resolved by discussion. To increase data collection homogeneity, outcomes were defined as follow: (1) SC-related complications were limited to ACS, painful crises, and neurological complications; (2) transfusion-related complications were limited to alloimmunization and transfusion reaction; (3) perioperative mortality; (4) postoperative infection; (5) complications related to surgery, including postoperative drop in hemoglobin >2 g/dl; (6) the number of transfused PRBCs (in units); (7) preoperative hemoglobin level in g/dl; and (8) preoperative HbSS percentage. Low-risk surgeries involved the eyes, nose, and ears, dental and distal extremities. Moderate-risk surgeries are those include orthopedic, genitourinary system, and intra-abdominal areas. High-risk surgeries are those involving the intracranial, cardiovascular, and intra-thoracic systems.
Data extraction
A standardized manual data extraction sheet was piloted and outcome definitions were discussed between the reviewers. The outcomes were clearly defined and written on the extraction sheet. Collected data included patient characteristics, treatment interventions, and outcomes. One reviewer collected the data and another verified the information. In case the data were not provided in the published paper or the online supplements, we contacted the corresponding authors.
Quality assessment and risk of bias
Relevant studies were selected and critically appraised using the criteria recommended by the US preventive services task force and the Canadian task force on preventive health care.Citation12,Citation13 Each study was labeled as good, fair, or poor on the basis of a priori established internal validity criteria. These predefined criteria used to assign the level of quality to RCTs and observational studies are summarized in Appendices 1 and 2, respectively. A web-based bibliographic tool (http://www.wizfolio.com) was used to download all references and ensure the absence of duplicates.
Data synthesis and analysis
The statistical analysis was conducted using the Review Manager Software (RevMan, version 5.1; from the Cochrane Collaboration, http://www.cochrane.org). For the meta-analysis of dichotomous outcomes, the estimated effect size and variation were expressed as relative risk and their respective 95% confidence interval (CI) for each study and for the pooled studies. For continuous outcomes, we used the mean difference (MD) and the 95% CI. In two studies, we estimated the standard deviations from a pertaining subgroup analysis paper published by the same author and for the same patients. Heterogeneity was assessed using the Q statistics and calculated using ICitation2 statistics to examine any possible heterogeneity between the studies. The random effect model assumption was used to adjust for within and between study heterogeneity. Forest plots were used to illustrate the individual studies, their final pooled effect size, and each individual study's weight (which is based on the inverse variance plus heterogeneity). We used common formulas to convert hemoglobin level to (g/dl) and unified transfused PRBCs units if reported in milliliter by dividing it by 250. If outcomes were reported as median, we estimated the mean by using standardized equations.Citation14 We planned to use funnel plots to assess publication bias if we had more than eight studies. A sensitivity analysis was conducted to show outcomes estimates by study design and shown in the corresponding Forest plot.
Results
Study selection and characteristics
Seventeen references (3 RCTs and 14 non-randomized controlled or observational studies) were identified by our search strategy. After examining the entire manuscripts, 10 studies were found to be suitable for meta-analysis (i.e. compared two interventions in two parallel groups) () and the other 7 studies were qualitatively reviewed ().
Table 2. Characteristics of studies not eligible for meta-analysis
Quality assessment and risk of bias
Three RCTs were included with variable quality. In one trial, the sample size was not sufficiently powered to detect all outcomes, however, this was due to the study being stopped early for safety concerns.Citation10 Al-Jaouni et al.Citation15 randomized patients to preoperative transfusion or no transfusion, and Vichinsky et al.Citation16 randomized patients to simple transfusion strategy or aggressive transfusion strategy, but neither reported the method of allocation, blinding, or the whether of not they used intention to treat analysis. Observational studies were poor in methodology and conducted with clear baseline variability and uncontrolled co-interventions. Quality assessment for included studies is summarized in .
Table 3. Summary of quality assessment for studies included in meta-analysis
Quantitative data synthesis and meta-analysis
Three RCTsCitation10,Citation15,Citation16 and seven cohorts studies published from 1995 to 2013 were analyzed. Four studies compared exchange transfusion to a simple transfusion regimen,Citation3,Citation16–Citation18 while the remaining compared preoperative transfusion strategy to no transfusion.Citation4,Citation10,Citation15,Citation19–Citation21 While one RCT included 551 patients,Citation16 overall studies were small with the number of patients included in the remaining studies ranging from 14 to 369. Surgical interventions included mild- and moderate-risk procedures, including tonsillectomies, orthopedic procedures, and abdominal surgery. None of the studies included high-risk surgeries.
Synthesis of results
Exchange versus simple blood transfusion
Perioperative mortality (n = 678)
One RCT and three observational studies evaluated perioperative mortality. Two participants died in the exchange transfusion arm compared to none in the simple transfusion arm, relative risk (RR) 4.91 (95% CI: 0.24–101.81, P = 0.30).
Serious complications related to SCD (n = 708)
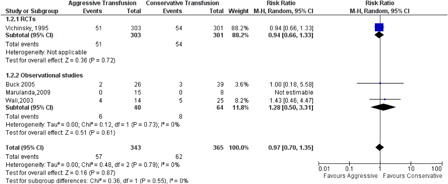
There was no statistically significant difference between the exchange and simple transfusion for SCD-related serious complications using a composite endpoint (ACS, painful crisis, and CVA; RR: 0.97, 95% CI: 0.70–1.35, P = 0.55, ).
Preoperative hemoglobin level (n = 666) and HbSS percentage (n = 666)
Three studies reported the preoperative hemoglobin level and the pooled MD between exchange and simple transfusion is 0.50 g/dl (95% CI: 0.26–0.75, P = 0.18). The preoperative HbSS% was lower in the exchange transfusion arm, with high heterogeneity ICitation2 = 92% mainly attributed to the observational studies since some of these studies are retrospective and the measurements were not recorded at the exact preoperative time interval. In the only RCT that reported the Hb S percentage after the intervention, the preoperative HbSS was 31 ± 16% in the exchange transfusion arm compared to 59 ± 15% in the simple arm, MD = −31.91% (95% CI: −43.13 to −20.70, P < 0.00001)
Major surgical complications (n = 731) and postoperative infections (n = 708)
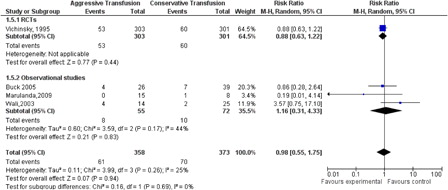
Analysis of surgical complications rate showed no statistical difference between exchange and simple transfusion with mild heterogeneity congruent with significant procedural heterogeneity RR 0.98 (95% CI: 0.55–1.75, P = 0.94, ). The distribution of the postoperative infections was similar RR 1.26 (95% CI: 0.64–2.47, P = 0.34).
Volume of blood transfused in units and transfusion-related complications (666)
The amount of blood transfused was higher in the exchange transfusion arm compared to the simple transfusion arm with 2.03 units MD (95% CI: 1.23–2.83, P < 00001). The heterogeneity was 98%. High-risk patients, defined as those with an American Society of Anesthesiologists score of 2 or 3, and those with previous SCD complications were aggressively transfused by full or partial exchange transfusions. The transfusion-related complications which include anaphylactic reactions were statistically significantly higher in the exchange transfusion group RR 2.41 (95% CI: 1.49–3.91, P = 0.0003)
Preoperative blood transfusion versus no transfusion
Perioperative mortality (n = 570)
Pooled data from two randomized studies and one observational study showed no difference in mortality between exchange and simple transfusion, with RR 0.19 (95% CI: 0.02–2.04, P = 0.17).
Serious complication related to SCD (n = 586)
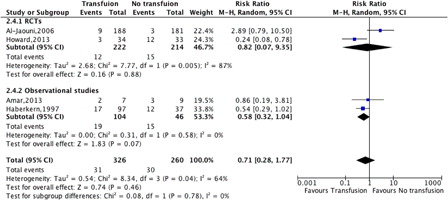
There was no difference between preoperative transfusion and no transfusion for the pooled estimate of ACS, painful crises, and SC-related neurological complications (RR 0.71, 95% CI: 0.28–1.77, P = 0.78, ).
Major surgical complications (n = 326) and postoperative infections (n = 492)
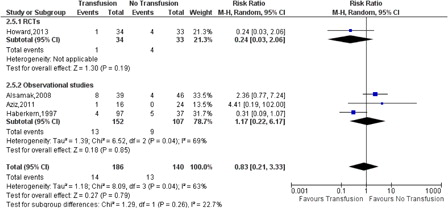
There was no difference between preoperative transfusion and no transfusion in the surgical complication rate (RR 0.83, 95% CI: 0.21–3.33, P = 0.26, ) or postoperative infection (RR 1.72, 95% CI: 0.57–5.25, P = 0.42).
Transfusion-related complications (n = 586)
There was no difference between preoperative transfusion and no transfusion for transfusion-related complications (RR 1.97, 95% CI: 0.58–6.6, P = 0.28).
Qualitative data review
Seven studies were identifiedCitation9,Citation22–Citation27 but were not eligible for meta-analysis because of the incomparability of between the groups or lack of comparative arm; therefore, they were included only in the qualitative analysis. They included patients from all age groups with different heterozygous sickle trait or homozygous SCD whom were scheduled for various surgical procedures. Most studies reported beneficial effect of preoperative transfusion-related mainly to the reduction of risk of postoperative complications. This effect was negligible in minor and low-risk procedures.
Discussion
In this study, we reviewed the literature evaluating exchange and simple transfusion in patients with SCD undergoing elective surgery. Only three randomized trial were available, and they were all underpowered to detect the main clinical outcomes. Exchange transfusion, compared to simple transfusion, and was found to be associated with higher use of transfused blood and higher incidence of transfusion reactions with no benefit in terms of perioperative mortality or postoperative complications. In addition, preoperative transfusion, using either simple or exchange transfusion or both, was not associated with protection from SCD-related adverse outcomes compared with no transfusion in patients undergoing low- to-moderate risk surgical procedures, but did expose patients to risks associated with transfusion. Our findings support those of the Preoperative Transfusion in Sickle Cell Group in 1995, who also concluded that exchange transfusion is no better than simple transfusion for preventing perioperative complications, and is associated with only half as many as transfusion-related complications.Citation4,Citation16,Citation27 Our findings also support the conclusions made by the Preoperative Transfusion in Sickle Cell Group for medium- and low-risk surgeries which showed that a conservative transfusion regimen is as effective as an aggressive regimen in preventing perioperative complications. Due to paucity of data, we were not able to assess transfusion regimens prior to high-risk surgeries, where the benefits could potentially be higher and thus the risks are more acceptable.
Our results were driven by two RCTs conducted in 2006 and 2013. Al-Jaouni et al. enrolled 369 stable SC anemia patients for low-to-medium risk surgeries and randomized them to either receive preoperative blood transfusion (simple or partial exchange) or no transfusion. Al-Jaouni et al.Citation15 found no advantage, in terms of reduced sickle-related complications, perioperative infection, and transfusion-related complications, but these findings are limited by lack of details on the used methodology and patient baseline characteristics. Howard et al. enrolled 70 patients for low- and medium-risk interventions,Citation10 and this study was stopped early due to a signal for harm in an interim safety analysis. There were higher SC-related complications in the no transfusion group, including ACS and acute painful crises, and the authors recommended preoperative transfusion to minimize these complications. Both studies had major quality and power limitations. Previous randomized trials had major obstacles with patient recruitment resulted in prolonged trial duration or early termination. Howard et al.Citation10 started recruiting patients from 2007 to 2011 and although they planned to recruit more than 400 patients to detect a difference of at least 10%, they were able to enroll only 70 patients and analyzed a mere 67. Again, none of the studies addressed high-risk surgery.
The vast majority of patients enrolled in the studies are of Hb S genotype and there is a lack of data regarding surgical interventions in patients with other genotypes. Neumayr et al.Citation25 recommended the use of preoperative transfusion in selected SC patients like patients undergoing abdominal surgery but unnecessary in minor procedures. In contrast, Koshy et al.Citation9 recommended the use of preoperative transfusion in all SC patients and for all procedures.
Limitations
Though we included the best data available, the included studies were frequently of poor quality and underpowered. Many confounders (such as use of hydroxyurea and hemoglobin F levels) were not adjusted for. Observational studies were very poor in quality and may not contribute to a generalizable body of evidence that can guide decisions about clinical practice. The previous SC complications, previous ACS, and hospitalizations should be taken into consideration and SC genotype should be equally distributed. These important factors were not always considered across studies. Though our study is limited by the quality of the studies in the literature, this review can be used as a background for further well-conducted studies. Perhaps the biggest limitation in the SC research area comes from the low recruitment rate in trials. SC anemia is not common in all parts of the world and surgical intervention is not always required. This might potentially be addressed by implementation of cluster randomized controlled design studies. Higher quality data are certainly required to answer the pertinent questions in this complex population of patients and further studies are required.
Conclusion
Based on the current literature, we found that preoperative transfusion, either with exchange transfusion or simple transfusion does not reduce perioperative mortality or perioperative complication rates in patients with SCD who are undergoing elective surgery. However, the overall quality of literature is low and studies are small, and so further research could alter the estimates of risk and benefit of these interventions.
Disclosure and competing interests statement
Dr M.S. McMurtry is funded by the Heart and Stroke Foundation of Canada. There are no other competing interests by any of the authors.
Acknowledgement
Alotaibi GS, MD, would like to acknowledge and thank the Saudi Arabian Ministry of Higher Education for funding his scholarship.
References
- Serjeant GR. The emerging understanding of sickle cell disease. Br J Haematol. 2001;112:3–18.
- Lovaglio PG. Patient safety analysis linking claims and administrative data. Int J Health Care Qual Assur. 2012;25:698–711.
- Buck J, Casbard A, Llewelyn C, Johnson T, Davies S, Williamson L. Preoperative transfusion in sickle cell disease: a survey of practice in England. Eur J Haematol. 2005;75:14–21.
- Haberkern CM, Neumayr LD, Orringer EP, Earles AN, Robertson SM, Black D, et al. Cholecystectomy in sickle cell anemia patients: perioperative outcome of 364 cases from the National Preoperative Transfusion Study. Preoperative Transfusion in Sickle Cell Disease Study Group. Blood 1997;89(5):1533–42.
- Chirikos TN, Roetzheim RG, McCarthy EP, Iezzoni LI. Cost disparities in lung cancer treatment by disability status, sex, and race. Disabil Health J. 2008;1:108–15.
- Vichinsky EP, Neumayr LD, Haberkern C, Earles AN, Eckman J, Koshy M, et al. The perioperative complication rate of orthopedic surgery in sickle cell disease: report of the National Sickle Cell Surgery Study Group. Am J Hematol. 1999;62:129–38.
- Dinan MA, Chou CH, Hammill BG, Graham FL, Schulman KA, Telen MJ, et al. Outcomes of inpatients with and without sickle cell disease after high-volume surgical procedures. Am J Hematol. 2009;84:703–9.
- Hirst C, Williamson L. Preoperative blood transfusions for sickle cell disease. Cochrane Database Syst Rev. 2012;1:pCD003149.
- Koshy M, Weiner SJ, Miller ST, Sleeper LA, Vichinsky E, Brown AK, et al. Surgery and anesthesia in sickle cell disease. Cooperative study of sickle cell diseases. Blood 1995;86:3676–84.
- Howard J, Malfroy M, Llewelyn C, Choo L, Hodge R, Johnson T, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013;381:930–8.
- Mateen FJ, Oh J, Tergas AI, Bhayani NH, Kamdar BB. Titles versus titles and abstracts for initial screening of articles for systematic reviews. Clin Epidemiol. 2013;6:89–95.
- Anonymous. Canadian Task Force on Preventive Health Care. 2009. http://www.ctfphc.org/.
- Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
- Al-Jaouni S A-MS, Qari M, Nawas MA, Abdel-Razeq H. The safety of avoiding transfusion preoperatively in patients with sickle cell hemoglobinopathies [abstract]. Blood 2002;100(11 Pt 2 of 2:21b).
- Vichinsky EP, Haberkern CM, Neumayr L, Earles AN, Black D, Koshy M, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med. 1995;333:206–13.
- Wali YA, al Okbi H, al Abri R. A comparison of two transfusion regimens in the perioperative management of children with sickle cell disease undergoing adenotonsillectomy. Pediatr Hematol Oncol. 2003;20:7–13.
- Marulanda GA, Minniti CP, Ulrich SD, Seyler TM, Mont MA. Perioperative management for orthopaedic patients with sickle cell anaemia. J Orthop Surg. (Hong Kong) 2009;17:346–50.
- Al-Samak ZM, Al-Falaki MM, Pasha AA. Assessment of perioperative transfusion therapy and complications in sickle cell disease patients undergoing surgery. Bahrain: College of Medicine and Medical Science, AGU; 2008.
- Ould Amar K, Rouvillain JL, Loko G. Perioperative transfusion management in patients with sickle cell anaemia undergoing a total hip arthroplasty. Is there a role of red-cell exchange transfusion? A retrospective study in the CHU of Fort-de-France Martinique. Transfusion clinique et biologique: journal de la Societe francaise de transfusion sanguine. 2013;20(1):30–4.
- Aziz AM, Meshikhes A-WN. Blood transfusion in patients with sickle cell disease requiring laparoscopic cholecystectomy. Saudi Arabia: Department of Surgery, King Fahad Specialist Hospital, Eastern Province; 2011.
- Bhattacharyya N, Wayne AS, Kevy SV, Shamberger RC. Perioperative management for cholecystectomy in sickle cell disease. J Pediatr Surg. 1993;28:72–5.
- Fu T, Corrigan NJ. Minor elective surgical procedures using general anesthesia in children with sickle cell anemia without pre-operative blood transfusion. 2005.
- Griffin TC, Buchanan GR. Elective surgery in children with sickle cell disease without preoperative blood transfusion. Dallas: Department of Pediatrics, University of Texas Southwestern Medical Center; 1993.
- Neumayr L, Koshy M, Haberkern C, Earles AN, Bellevue R, Hassell K, et al. Surgery in patients with hemoglobin SC disease. Preoperative Transfusion in Sickle Cell Disease Study Group. California, USA: Department of Hematology/Oncology, Children's Hospital Oakland; 1998.
- Augier R, Tennant IA, Reid M, Harding H, Crawford-Sykes A, Bortolusso-Ali S, et al. Perioperative transfusion of patients with sickle cell disease undergoing surgery at the University Hospital of the West Indies (UHWI) [Online]. 2008. http://ispub.com/IJA/21/2/11517 (accessed 13 Feb 2014).
- Waldron P, Pegelow C, Neumayr L, Haberkern C, Earles A, Wesman R, et al. Tonsillectomy, adenoidectomy, and myringotomy in sickle cell disease: perioperative morbidity. Preoperative Transfusion in Sickle Cell Disease Study Group. J Pediatr Hematol Oncol. 1999;21:129–35.