Abstract
Objective
Platelet (P) and endothelial (E) microparticle (MP) levels increase in preeclampsia. However, their relation to the severity of the disease needs to be clarified. The objectives of this study were to compare the levels of EMP and PMP in severe and mild preeclampsia to healthy gravidas to find possible correlations to severity of the disease, Doppler changes, and complications.
Methods
A comparative prospective clinical trial (Canadian Task Force II-1) was conducted on 135 pregnant women divided into three groups: 35 women with severe preeclampsia (group 1), 40 with mild preeclampsia (group 2), and 60 healthy gravids (group 3). Assessment of EMP and PMP was done by flow cytometry using anti-CD31 and anti-CD42b antibodies.
Results
Expression of CD31 and CD42b (EMPs) was higher in group 1 compared to groups 2 and 3 with P < 0.001, while expression of CD42b alone (PMPs) did not show a statistically significant difference (P = 0.957). EMPs were correlated positively to umbilical and middle cerebral artery resistance index. There was a significant negative correlation between platelet count and EMPs. Also, EMPs were correlated positively to aspartate transferase and bilirubin levels and were significantly higher with neonatal death.
Discussion
The present study revealed a significant association between plasma levels of EMPs and severity of preeclampsia together with poor neonatal outcome as regards birth weight and percent of neonatal death. So, EMPs assay could be a good predictor of maternal and fetal outcomes and in cases with preeclampsia.
Introduction
Membrane vesiculation is a general physiological process that leads to the release of cell plasma fragments, called microparticles (MPs).Citation1–Citation3 These MPs can be generated from nearly every type of cell during activation, injury, or apoptotic processes.Citation1 All MPs, whatever their cell origin, have negatively charged phospholipids (e.g. phosphatidylserine) in their outer membrane leaflet, accounting for their procoagulant properties.Citation3 They also express proteins, characteristic of their cellular origin, on their surface and carry proteins packaged from numerous cellular compartments.Citation4,Citation5 MPs can participate in the maintenance of homeostasis under physiological conditions. Among the various circulating MPs, platelet-derived MPs are the most abundant in the bloodstream, representing between 70 and 90% of circulating MPs in healthy subjects.Citation6
Vascular endothelium aggression may lead to the vesiculation and shedding of endothelial MPs (EMPs). EMPs could be used as a marker of endothelial injury, directly reflecting the state of the homeostasis between endothelial cell activation, proliferation, and apoptosis.Citation7 Accumulating evidence shows that circulating EMP levels were drastically increased in several pathological conditions related to endothelial impairments such as hypertension, dyslipidemia, diabetes mellitus, coronary artery disease, thrombotic thrombocytopenic purpura, heart failure, renal failure, graft-versus-host disease after hematopoietic cell transplantation, and systemic lupus erythematosus.Citation8–Citation19 These data emphasize the relation between endothelial damage, the release of EMP, and the modulation of inflammatory and/or immune responses.
Preeclampsia is one of the most significant health problems of human pregnancy. It is a leading cause of fetal growth restriction, premature birth, and low birth weight babies.Citation20 In Egypt, the national survey for maternal mortality ranked preeclampsia/eclampsia as the second cause of maternal death.Citation21
Although recognized by hypertension and proteinuria, preeclampsia is much more than these two changes. A predominant pathophysiological feature is the reduced perfusion of virtually all organs that is due to vasoconstriction, microthrombi formation, and reduced circulating plasma volume.Citation21
As important evidence existed to implicate endothelial cell injury to the pathophysiology of this pregnancy-specific disease, the present study was designed to determine the possible association between the levels of plasma EMPs and platelet MPs (PMPs) of pregnant women with preeclampsia and the disease severity, together with its relation to neonatal outcome.
Patients and methods
The present study was conducted on 75 women with preeclampsia (age mean ± SD: 26.5 ± 5.7), and 60 healthy pregnant women (age mean ± SD: 26.1 ± 3.4) at >34 weeks of gestation who were recruited to El-Kasr El-Aini Hospital from September 2011 to December 2012. Each woman provided informed consent before study enrollment. The criteria used for diagnosis and classification of preeclampsia were defined according to The Report of The National High Blood Pressure Education Program Working Group, 2000 by hypertension ≥140/90 with proteinuria ≥300 mg of urinary protein per 24 hours or persistent 30 mg/dl (1 + dipstick) in random urine samples after the 20th week of gestation.
Patients were classified according to severity of preeclampsia into the severe (35 patients) and mild group (40 patients). Severe preeclampsia was defined as women with blood pressure of >160/110 mm Hg in two determinations 4 hours apart and proteinuria ≥2 g/24 hours, platelet count of <100 000 cells/cmm, or HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count). Only two cases were diagnosed as having HELLP syndrome. The mild group was defined by blood pressure ≥140/90 and <160/110 mm Hg in two determinations 4 hours apart, and proteinuria ≥300 and <500 mg/24 hours. Patients with chronic hypertension, pre-gestational diabetes mellitus, and history of recurrent pregnancy loss, renal or hepatic diseases, as well as antiphospholipid syndrome and women in labor were excluded.
All studied groups were subjected to history taking, laboratory investigations including complete blood count, using EDTA-anticoagulated blood assayed on Beckman Coulter (Brea, California, USA) and aspartate transferase (AST), using serum samples assayed on Hitachi 912 with kits provided by Roche Diagnostics (Mannheim, Germany) to diagnose cases with HELLP syndrome, then obstetric ultrasound sound examination was performed by one operator (W.S.) using 5.0 MHz curvilinear transabdominal transducer (Toshiba ECCO CEE SSA-340 A), to assess gestational age through determination of the fetal biparietal diameter, abdominal circumference, femur length, and the estimated fetal weight while the pregnant women was in a slightly tilted semi-fowler position with the head of the bed raised 30° and with a small pillow under the right loin the instrument was used. Doppler velocimetric studies were done for all participants to calculate the systolic/diastolic ratio, the presence and laterality of the diastolic notch, pulsatility index (PI), and resistance index (RI) of both umbilical and middle cerebral arteries.
For the umbilical artery Doppler, an area of the amniotic cavity with several loops of umbilical cord was selected; using pulsated wave Doppler, the characteristic sound and shape of the umbilical artery waveform were demonstrated and identified. When the screen showed several waveforms of similar height, the image was frozen and the peak systolic frequency, end diastolic frequency, S/D ratio, and PI were estimated. A minimum of three separate readings were averaged before the final values were obtained. The middle cerebral artery (MCA) was visualized near its distal (straight) portion. When the MCA pattern was imaged, it was freeze framed and the RI was calculated. Because of the potential effect of fetal breathing movements on waveform variability, recording was performed during periods of fetal apnea. Taking into consideration that the normal umbilical artery third trimester S/D ratio is 2.5 ± 0.4 with a cutoff value of 3 and PI is 0.89 ± 0.12 with cutoff value 1.1,Citation9 the Doppler index values were obtained and averaged to determine the RI used in the calculations. The cerebroplacental ratio (CPR), defined as the MCA-RI divided by the umbilical artery (UA)-RI, was considered abnormal if <1.0.Citation22
After delivery, neonatal birth weight, Apgar score at 1 and 5 minutes, and neonatal intensive care unit admission were recorded.
Assay of circulating EMPs by flow cytometry
After informed consents were obtained, blood samples were collected by clean venipuncture into 2 ml vacutainer tubes containing sodium citrate from cases and controls under strict sterile conditions. The samples were centrifuged 20 minutes at 800 rpm to prepare platelet-rich plasma (PRP). PRP was centrifuged 5 minutes at 5000 rpm to obtain platelet-poor plasma (PPP) and to remove all the residual platelets or cell fragments of a similar size. The samples were assayed within 4 hours of venipuncture to avoid contamination with MPs that are released ex vivo.
EMP and PMP immunolabeling
Fluorescein isothiocyanate dye (FITC) labeled mouse antihuman CD31 and phenylethylamine (PE) mouse anti-human CD42b, Serotec (Kidlington, UK), were used to detect EMPs and PMPs. Fifty microliters of PPP was incubated with 5 ml of each labeled antibodies in the dark at room temperature for 20 minutes. Tubes were washed twice with 2 ml 0.01 mol/l phosphate-buffered saline (PBS), pH 7.3, and then centrifuged at 2000 rpm for 5 minutes, supernatants were aspirated. The cells were resuspended in 0.5 ml of PBS. The stained samples were finally mixed and kept for flow cytometry analysis. Non-immunized FITC- and PE-labeled isotype-matched mouse monoclonal IgG was used as control, Serotec. The mean fluorescence intensity (MFI) ratio was obtained by dividing the MFI for a given marker by the MFI of the respective isotype control monoclonal antibody.
Flow cytometry analysis
Assay of levels of MPs was done using Beckman Coulter Epics XL, USA. The light scatters and fluorescence channels were set at logarithmic gain. Regions corresponding to cells or shed MPs were gated on separate protocols, using forward light scatter (FSC) which is roughly equivalent to particle size versus side-angle light scatter (SSC) proportional to the granularity of studying cells. Microparticles were analyzed regarding size and fluorescence. Briefly, on a LogFS–LogSS dot plot, the upper size limit of the MPs was defined using 1 µm calibrant beads, and a gate was drawn around the population. The lower size limit of the gate excludes the first channels that contain the electronic background noise of the machine. Only the events included in this gate were further analyzed for their fluorescence on a LogSS–LogFL dot plot. Assay of MFI of the positive cell populations, only included in the gate, was measured for each antigen. Logarithmic green and red fluorescence of FITC and PE were picked up by the detectors: photomultiplier tube 2 (PMT2) and PMT3, respectively ( and ).
Figure 1. Histograms of the negative control samples for FITC- (A) and PE- (B) labeled isotypic antibodies. B-line was drawn to exclude non-specific signals released by FITC negative control. C-line was drawn to exclude non-specific signals released by PE negative control. (C) shows that all particles were presented in quadrant 3 (negative signals for both FITC- and PE-labeled isotypic antibodies.
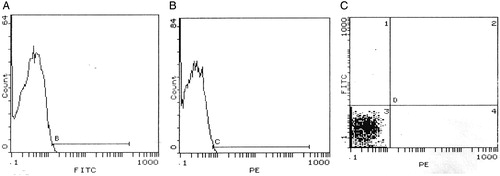
Figure 2. Histograms of flow cytometry show a case positive for both CD31 (A) and CD42b (B). (C) represents a four-quadrant histogram to detect co-expression of both markers (quadrant 2), i.e. PMP, while quadrant 1 represents MPs positive only for CD31, i.e. EMPs.
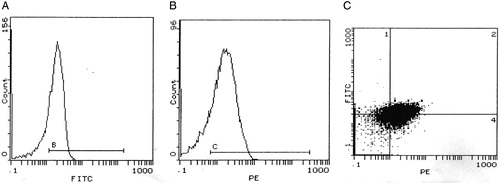
Before every test, the machine was kept running until the background events fell to baseline levels that could be neglected. Four quadrants histogram was used to differentiate EMPs, being positive for CD42b and negative for CD31, from PMPs, being positive for both CD42b and CD31.The exact numbers of MPs were estimated by using calibrated latex microbeads (Sigma Aldrich, St Louis, Missouri, USA) that were added to samples just before analysis. The diameter of the beads (3 µm) discriminated them from the MP population on the FSC–SSC histogram. A known number of beads were added to each sample tube, and the analysis was stopped when 10 000 beads were reached. The number of MPs per microliter plasma was calculated with the formula N = (MP/number of measured beads) × (beads per tube/volume of plasma).
Statistical analysis
Data were described in terms of mean ±SD, median, and range whenever appropriate. Comparison between the different groups of the study was done using Student's t-test for comparing continuous data when normally distributed and Mann–Whitney U test when not normally distributed. For comparing categorical data, Chi square (cCitation2) test was performed. Correlation between various variables will be done using Pearson moment correlation coefficient (r). A probability value (P value) <0.05 was considered significant. All statistical calculations were done using computer programs Microsoft Excel version 7 (Microsoft Corporation, New York, NY, USA) and SPSS for Windows version 13.0 (Statistical package for the Social Science; SPSS Inc., Chicago, IL, USA).
Results
By definition, values of systolic and diastolic blood pressure were higher in the severe preeclampsia group compared to mild cases, and both groups were higher compared to the control group. Control women were negative for albuminuria, while +3 and +4 albumin was detected in the urine of severe preeclamptic women. The demographic and laboratory data of the participants are summarized in Tables and .
Table 1. Showing demographic and laboratory data of the three groups
Table 2. Results of blood pressure and albumin of the three groups
As concern results of Doppler study of middle cerebral and umbilical arteries, in both arteries, the RI and PI were significantly higher in the severe preeclampsia group compared to the mild preeclampsia and control groups. Also, RI and PI of the two arteries were significantly higher in the mild preeclampsia group in comparison to the control group. Diastolic notch were significantly more frequent in the severe preeclampsia group. Neonatal outcome of the studied groups are summarized in .
Table 3. Neonatal outcome of the three studied groups
Results of EMPs and PMPs levels in the studied groups
EMPs counts were significantly higher in the severe preeclampsia group compared to the other two groups and in the mild preeclampsia group in comparison to the control group. On the other hand, PMP (PMP) counts showed no significant difference between the three studied groups (). No significant correlation was found between EMPs and PMPs (r = 0.066, P = 0.582).
Table 4. EMPs and PMPs count in the three studied groups
There was a significant positive correlation between systolic and diastolic blood pressure and the EMPs level (P < 0.001). However, EMPs level was not correlated with age of the mothers. PMPs count did not show any correlation with age or blood pressure. There was a significant positive correlation between RI and PI of the middle cerebral artery and EMP (RI (r = 0.374, P < 0.001) and PI (r = 0.345, P < 0.003)). Also, EMPs were correlated positively to umbilical artery RI but not to umbilical artery PI (RI (r = 0.424, P < 0.001); PI (r = 0.182, P = 0.128)). Contrarily, PMPs count was not correlated to Doppler parameters.
EMPs levels were correlated positively to AST values (r = 0.291and P = 0.014). Meanwhile, EMPs and PMPs counts were not correlated to other laboratory parameters (hemoglobin levels and platelet counts).
As regards neonatal birth weight, gestational age at delivery, and Apgar score, there was a significant negative correlation between neonatal birth weight and EMPs (P = 0.004, r = −0.339). However, EMPs was not correlated to gestational age at delivery or Apgar score at 5 minutes. PMPs count was not correlated to neonatal parameters.
As regards neonatal outcome, EMPs were significantly higher in cases with neonatal death in comparison to those with living neonates (P = 0.044). There was no significant difference between group with neonatal death and those with free neonates regarding PMPs.
Discussion
The central feature in the pathogenesis of preeclampsia is a generalized vascular dysfunction. EMPs are vesicles that are derived from the endothelial surface itself. When budding occurs as result of endothelial insult, the surface marker CD31 is expressed on the surface of EMP. Identification of these MPs has provided direct evidence of endothelial damage in preeclampsia and helped to distinguish preeclampsia from gestational hypertension.Citation23
The present study provided correlation between severity of preeclampsia and EMP levels, which showed a statistically significant difference in preeclamptic patients as compared to the control group and in severe preeclampsia in comparison to the mild group (P < 0.001). The previous results suggest EMP levels as an indicator of severity of hypertension, which is likely indicative of progressive endothelial damage. This supports the previous findings in which non-pregnant patients with severe hypertension exhibited elevated EMP levels when compared with the mild hypertension and the control groups.Citation24 As regards PMPs, there was no significant difference in their counts between the three studied groups (P = 0.957).
These results are in agreement with that of Gonzalez-Quintero et al.,Citation23 who studied the levels of EMPs in 52 women with preeclampsia in comparison to 20 patients with gestational hypertension and 38 healthy control groups, and found highest levels to be associated with preeclampsia. EMP also exhibited higher levels, in relation to the severity of the disease.
The scientific plausibility of these results supports the endothelial injury theory in preeclampsia and may be useful as an early indicator of preeclampsia in pregnant women.
No correlation has been observed between the total numbers of MPs and the age in each studied group (P = 0.140). Likewise, no data in the literature suggest any impact of age on MP levels except in subjects younger than 18 years old.Citation25
EMPs were significantly correlated to maternal serum AST (P = 0.014). This observation which was not reported before is in favor of the theory incriminates EMPs in the pathogenesis of vascular dysfunction in preeclampsia.
EMPs are significantly correlated to middle cerebral artery RI and PI (RI (r = 0.374, P < 0.001) and PI (r = 0.345, P < 0.003)). EMPs are also significantly correlated positively to umbilical RI but not to umbilical PI. RI (r = 0.424, P < 0.001); PI (r = 0.182, P = 0.128). PMPs count was not correlated to Doppler parameters. Abnormal uterine Doppler is explained by lack of normal trophoblastic invasion in preeclamptic patients. It is considered as an indicator of poor uteroplacental circulation which affects the perinatal outcome.
This results support the hypothesis that EMPs may be involved in the pathogenesis of preeclampsia.
Higher EMPs are significantly correlated to poor neonatal outcome as low neonatal birth weight and neonatal death (P = 0.004 and 0.044, respectively) but PMP levels were not correlated to neonatal parameters.
Conclusion
The present study revealed a significant association between plasma levels of EMPs and severity of preeclampsia together with poor neonatal outcome as regards birth weight and percent of neonatal death. More studies are needed to demonstrate levels of EMPs in different weeks of pregnancy to assess its value as an early marker for diagnosis of preeclampsia.
Disclaimer statements
Contributors All authors certify that they have participated sufficiently in the intellectual content.
Funding None.
Conflicts of interest None.
Ethics approval Approval was taken from the local ethics committee.
References
- Ardoin SP, Shanahan JC, Pisetsky DS. The role of microparticles in inflammation and thrombosis. Scand J Immunol. 2007;66:159–65.
- Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–7.
- Leroyer AS, Tedgui A, Boulanger CM. Role of microparticles in atherothrombosis. J Intern Med. 2008;263:528–37.
- Banfi C, Brioschi M, Wait R, Begum S, Gianazza E, Pirillo A, et al. Proteome of endothelial cell-derived procoagulant microparticles. Proteomics 2005;5:4443–55.
- Peterson DB, Sander T, Kaul S, Wakim BT, Halligan B, Twigger S, et al. Comparative proteomic analysis of PAI-1 and TNF-α-derived endothelial microparticles. Proteomics 2008;8:2430–46.
- Leroyer AS, Isobe H, Leseche G, Castier Y, Wassef M, Mallat Z, et al. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49:772–7.
- Horstman LL, Jy W, Jimenez JJ, et al. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci. 2004;9:1118–35.
- Preston RA, Jy W, Jimenez JJ, et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003;41:211–7.
- Koga H, Sugiyama S, Kugiyama K, et al. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–30.
- Bernal-Mizrachi L, Jy W, Jimenez JJ, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–70.
- Heloire F, Weill B, Weber S, et al. Aggregates of endothelial microparticles and platelets circulate in peripheral blood. Variations during stable coronary disease and acute myocardial infarction. Thromb Res. 2003;110:173–80.
- Jimenez JJ, Jy W, Mauro LM, et al.. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001;112:81–90.
- Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–8.
- Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, et al. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006;4:566–73.
- Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG, et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes 2002;51:2840–5.
- Pihusch V, Rank A, Steber R, Pihusch M, Pihusch R, Toth B, et al.. Endothelial cell-derived microparticles in allogeneic hematopoietic stem cell recipients. Transplantation 2006;81:1405–9.
- Nomura S, Ishii K, Inami N, Kimura Y, Uoshima N, Ishida H, et al. Evaluation of angiopoietins and cell-derived microparticles after stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:766–74.
- Joseph JE, Harrison P, Mackie IJ, Isenberg DA, Machin SJ. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br J Haematol. 2001;115:451–9.
- McNiff JM, Kaplan DH. Plasmacytoid dendritic cells are present in cutaneous dermatomyositis lesions in a pattern distinct from lupus erythematosus. J Cutan Pathol. 2008;35:452–6.
- Walsh SW, Vaughan JE, Wang Y, et al. Placental isoprostane is significantly increased in pre-eclampsia. FASEB J. 2000;14:1289–96.
- Granger JP, Alexander BT, Llinas MT, et al. Pathophysiology of hypertension during pre-eclampsia linking placental ischemia with endothelial dysfunction. Hypertension 2001;38(3):718–28.
- Arias F. Accuracy of the middle-cerebral-to-umbilical-artery resistance index ratio in the prediction of neonatal outcome in patients at high risk for fetal and neonatal complications. Am J Obstet Gynecol. 1994;171(6):1541–5.
- Gonzalez-Quintero VH, Jimenez JJ, Smarkusky LP, Mauro LM, Jy W, Hortman L, et al. Elevated plasma endothelial microparticles: pre-eclampsia versus gestational hypertension. Am J Obstet Gynecol. 2004;191:1418–24.
- Mallat Z, Banamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, et al. Elevated levels of shed membrane microparticles in procoagulant potential in the peripheral circulating blood of patients with acute coronary syndrome. Circulation 2000;101:841–3.
- Proulle V, Hugel B, Guillet B, Grunebaum L, Lambert T, Freyssinet JM, et al.: Circulating microparticles are elevated in haemophiliacs and non-haemophilic individuals aged <18 years. Br J Haematol. 2005;131:487–9.
