Abstract
Background/Objectives
Beta (β)-thalassemia adults are prone to premature atherosclerosis but data about this complication among thalassemia children are few. Lipoprotein-associated phospholipase A2 (Lp-PLA2) and tumor necrosis factor-α (TNF-α) are inflammatory markers that could be implicated in atherosclerotic process. We investigated Lp-PLA2 and TNF-α levels in β-thalassemia children and their relation to subclinical atherosclerosis.
Methods
Twenty-two β-thalassemia major (TM), 20 β-thalassemia intermedia children, and 30 age- and sex-matched healthy controls were included. Lipid profile (by colorimetric assay), serum ferritin, TNF-α, and plasma Lp-PLA2 levels (by enzyme-linked immunosorbent assay technique) were estimated. Carotid intima-media thickness (cIMT) was measured by high-resolution ultrasound.
Results
Both patient groups exhibited anti-atherogenic lipid profile except increased serum triglycerides. They had significantly higher plasma Lp-PLA2 and serum TNF-α compared to the controls (P < 0.001). Elevated cIMT was documented in 57% of the thalassemia children and was higher among hepatitis C (HCV) positive patients. Serum ferritin, TNF-α, and plasma Lp-PLA2 levels were significantly higher in patients with premature atherosclerosis. cIMT correlated significantly with serum ferritin, TNF-α, and plasma Lp-PLA2 in both patient groups. Among TM children, serum ferritin had significant positive correlation with serum TNF-α and plasma Lp-PLA2. The elevation of both markers was not related to HCV infection.
Conclusions
Premature atherosclerosis is common among young thalassemia children. Lp-PLA2 and TNF-α are significantly increased in thalassemia children and show strong correlations with cIMT, suggesting that both of them may be appreciated as modulating factors in carotid atherosclerosis pathophysiological process among these children.
Introduction
Beta (β) thalassemia is an inherited hemoglobin (Hb) disorder characterized by defective β-globin chain synthesis. It has a diverse wide range of severity including transfusion-dependent thalassemia major (TM) and non-transfusion-dependent thalassemia intermedia (TI).Citation1
Thromboembolic events specially strokes are considered one of the serious β-thalassemia complications suggesting a pro-atherogenic liability in these patients.Citation2 In this regard, vascular endothelial abnormalities together with vasomotor dysfunctions and increased arterial stiffness were documented in β-TM as an early predisposing pathological abnormalities for atherosclerosis.Citation3,Citation4 In addition to endothelial dysfunctions and activation, arterial elastic tissue injury and enhanced pro-coagulant activity of the red cells have been demonstrated in β-TI patients.Citation5,Citation6
There is a growing body of evidence that considers atherosclerosis as low-grade chronic inflammatory disease of the arterial wall rather than a mere passive lipid accumulation. Inflammatory process is essential for the initiation and progression of vascular remodeling.Citation7 Accumulated lipid interaction with various inflammatory cells specially monocytes/macrophages and T-cells within the vessel wall is the main pathophysiological process of atherosclerosis.Citation8
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a pro-inflammatory enzyme preferentially secreted by monocytes, macrophages, neutrophils, and activated platelets in the arterial wall and circulates in plasma associated with lipoproteins.Citation9 It exhibits Ca2+-independent phospholipase A2 activity that could exert pro-atherogenic function through hydrolysis of oxidized phospholipids (oxPLs) generating pro-inflammatory oxidized, non-esterified fatty acids and lysophosphatidylcholine which are accumulated in the arterial wall eliciting several deleterious inflammatory responses involved in the pathobiology of atherosclerosis.Citation10
The inflammatory component of atherosclerosis is also mediated by several cytokines including tumor necrosis factor alpha (TNF-α)Citation11 that has a crucial role in atherosclerotic inflammatory cascade through endothelial cell activation, adhesion molecule expression up-regulation, and vascular remodeling with increasing arterial stiffness.Citation8
Carotid artery intima-media thickness (cIMT) is a reliable non-invasive well-validated marker of generalized subclinical atherosclerosis.Citation12 It is well correlated with vascular risk factors and can predict future atherosclerosis-related cardio-cerebrovascular events.Citation13
Increased cIMT as an indicator of premature (subclinical) atherosclerosis had been documented in adult and adolescent β-thalassemia patients.Citation14–Citation18 However, the published data about this complication in β-thalassemia children are scarce.Citation19,Citation20 Furthermore, to the best of our knowledge, no published data are available about the relation between Lp-PLA2 and TNF-α and premature atherosclerosis in β-thalassemia children. Therefore, considering the hypothesis that thalassemia children are exposed to the atherosclerotic risk factors like iron overload and increased oxidative stress, we investigated the presence of premature atherosclerosis among these children using cIMT illustrating its relation to Lp-PLA2, TNF-α (as inflammatory markers), and other biochemical risks as iron overload and lipid profiles.
Subjects and methods
Subjects
This study included 42 thalassemia children recruited from the Pediatric Hematology Clinic, Menoufia University Hospital, Egypt, attending for regular follow-up and red cell transfusion. Thirty normal age-, sex-, surface area-matched healthy children were involved as controls. The studied children were categorized into three groups.
Group (I): Included 22 β-TM children (13 males and 9 females) with their age ranging from 3 to 18 years (mean of 9.8 ± 5.2 years, median of 9.5 years) and disease duration ranging from 2 to 17.5 years (mean of 8.9 ± 5.1 years, median of 8.7 years). These patients were on a regular blood transfusion regimen since their first year of life to maintain pre-transfusion Hb above 7.5 g/dl and post-transfusion Hb above 10 g/dl.
Group (II): Included 20 β-TI children (10 males and 10 females) with their age ranging from 4 to 18 years (mean of 11.8 ± 4.6 years, median of 11 years) and disease duration ranging from 1 to 13 years (mean of 7.2 ± 3.6 years, median of 8 years). These patients had received only sporadic blood transfusions (less than four times each year).
Chelation therapy was usually started when serum ferritin approximated 1000 ng/ml.Citation21 According to this, 12 TM children received regular chelation therapy with subcutaneous Deferoxamine (DFO) infusion in a dose of 30–50 mg/kg/day, 5 days/week. Nine children were on regular oral chelation with deferasirox (20–30 mg/kg/day) and the remaining children were under combined therapy of both; DFO 3 days/week and daily oral deferasirox.
For TI children, 5 were on regular DFO therapy, 13 received oral deferasirox by the same dose like TM patients while 2 children did not need to start the chelation therapy (serum ferritin below 1000 µg/ml). Compliance with chelation therapy was considered satisfactory if the mean index of observation (OI) (the actual received DFO dose/the prescribed DFO dose × 100) was ≥80%.Citation22
All included patients were non-smokers and free from acute illness including infections with normal echocardiographic findings (normal biventricular systolic functions and pulmonary artery pressure). Patients with hypertension (>90th percentile according to age, sex, and height), obesity (body mass index (BMI) >95 percentile for age and sex), diabetes mellitus, hypothyroidism, active hepatic diseases, chronic renal disease, chronic vascular disease, history of familial hypercholesterolemia, or family history of coronary artery disease were excluded.
Group (III): Included 30 age-, sex-, surface area-, and socioeconomic standard-matched healthy children (10 males and 20 females) with their age ranging from 3 to 18 years (mean of 11.7 ± 6.8 years, median of 15 years). They were non-smokers with normal complete blood count (CBC), Hb electrophoresis and lipid profiles and no family history of dyslipidemia. They were recruited from children presented to our general pediatric clinic for well-being check-up.
The study was performed in the period from October 2012 to October 2013. Informed consent was obtained from the legal guardians of the included children and ethical clearance from the ethical committee in our medical school was obtained before study beginning.
Methods
Clinico-demographic data of the included patients were collected including disease duration, transfusion history (including age of first transfusion, frequency, and amount) with the calculation of transfusion index estimated as amount of transfused packed red cells in ml/kg body weight/year, chelation history (type, duration, and compliance), and history of operation specially splenectomy.
For all included children (patients and controls), weight and height were measured by the standard methods with the estimation of surface area and BMI (BMI = weight in kg/height in mCitation2). Pulse rate recording and blood pressure measurement using the suitable cuff size according to the arm length were also done.
CBC was obtained using AC920 Autocounter after calibration. Pre-transfusion values were considered for thalassemia patients who were due for red cell transfusion.
The mean yearly serum ferritin level in the previous year was considered (on the average of four determinations) for each patient. For the controls, single estimation of serum ferritin was done at the same time of sampling. Serum ferritin was measured by enzyme-linked immunosorbent assay (ELISA) technique (BioRad, Hercules, CA, USA).
Hepatitis C and B markers by polymerase chain reaction technique were done for patients and controls.
Sample collection and assay for other biochemical analyses
Fasting (10–12 hours overnight) venous blood samples were drawn by sterile vein puncture. In patients receiving blood transfusion, pre-transfusion samples were considered. Each sample was divided into two tubes, EDTA tube (to obtain plasma) and plain tube (to obtain serum). Blood samples were immediately centrifuged for 15 minutes at 3000 rpm; plasma and sera were separated and then were stored at −20°C until analysis. The serum aliquot was used for enzymatic colorimetric determination of blood urea, serum creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC),Citation23 serum triglycerides,Citation24 and high-density lipoprotein cholesterol (HDL-C).Citation25 Serum lipids were measured using Spinreact Kit (Girona, Spain). Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald formula.Citation26
Serum TNF-α and plasma Lp-PLA2 levels were measured by ELISA using AssayMax Human TNF-α kit (Assaypro, St Charles, MO, USA) and Lp-PLA2 Kit (Glory Science Co., Ltd, Del Rio, TX 78840, USA), respectively, according to the manufacturer's protocols.
Ultrasound assessment of cIMT measurement
Intima-media thickness (IMT) of carotid arteries was assessed by ultrasonography. The left and right carotid arteries were examined in multiple directions. The beginning of the dilatation of the carotid bulb was used as a reference point for measurement of the IMT. IMT was defined as the distance between the boundaries of lumen–intima and media–adventitia interfaces at the far common carotid artery (CCA) wall. The subjects were studied in the morning under standardized conditions (quiet room, comfortable temperature) with the participant in the fasting state. Blood pressure was measured under resting conditions. High-resolution ultrasonography was performed with a Hitachi 70 000 high-resolution ultrasound machine equipped with a linear array 12.5 MHz transducer. The participants were examined in the supine position with the head turned slightly to both sides. After identifying the bulb, longitudinal images of the CCA were obtained by combined B-mode and color Doppler. The scan was focused on the far wall and the resolution box was used to magnify the far wall segment 10–20 mm proximal of the carotid bulb, where all measurements were done. Several images were acquired by using an anterior oblique angle (30° from midline) and lateral angle (100° from midline). The IMT of the far wall was measured. Three scans on both sides were selected and nine (3 × 3) measurements of maximum far wall IMT on both sides were averaged; thus, the conclusive mean IMT of each patient was calculated from a total of 18 measurements. Plaque was defined as a focal structure encroaching into the arterial lumen by at least 0.5 mm or 50% of the surrounding IMT.
Statistical method
The data were processed on an IBM-PC compatible computer using SPSS version 18 (SPSS Inc., Chicago, IL, USA). Continuous parametric variables were presented as means ± SD, while for categorical variables numbers (%) were used. In statistical analyses, compatibility with normal distribution was evaluated using Shapiro–Wilk normality test. Chi-square test was used for qualitative variables. The difference between two groups was performed by student's t-test for parametric continuous variables and by Mann–Whitney (U) test for non-parametric variables. For more than two groups, one-way analysis of variance test was used for parametric data and the Kruskal–Wallis test was applied to discriminate differences in non-parametric variables. The least significant difference test (for parametric variables) and Tukey's honest significant difference (HSD) test (for non-parametric variables) were applied for comparisons between individual groups when appropriate. Pearson correlation (r): was the test used to measure the association between two quantitative parametric variables and Spearman correlation coefficient was applied for non-parametric data. P-value of <0.05 was considered statistically significant.
Results
Study population characteristics
The baseline clinical characteristics were similar in patient groups compared with the controls except for the BMI that was significantly lower and the heart rate that was significantly higher in both patient groups compared with the control group. About 41% of TM children and 50% of TI group had been splenectomized between the age of 5 and 12 years. Hepatitis C viral (HCV) infection was detected in 45.5 and 40% of TM and TI patients, respectively, while only one TM patient was found to have Hepatitis B viral infection (all of low viral load). All children of the control group were free from both infections. Seventeen out of the 22 TM patients (77%) were well chelated, while in the TI group this was true for 11 out of the chelated 18 patients (61%). Children with TM needed to be transfused by significantly higher amount of packed red cells compared with children of TI ().
Table 1. Characteristics of the studied groups
Hematological and biochemical parameters
Both thalassemia groups exhibited significantly lower pre-transfusion Hb compared with the basal Hb level of the controls. The median values for serum ferritin were as follows: 2176 ng/ml (range, 494–13 000 ng/ml), 2405 ng/ml (range, 720–10 000 ng/ml), and 97 ng/ml (range, 76–130 ng/ml) for TM, TI children, and the controls, respectively. The mean yearly serum ferritin levels in both thalassemia groups were significantly elevated compared with the single estimate in the control group without significant difference between both patient groups. No significant difference was observed between the three groups in terms of blood urea or serum creatinine (P > 0.05). On the other hand, both patient groups had significantly elevated liver transaminases levels. Compared to controls, both patient groups demonstrated significantly lower serum TC, LDL-C, HDL-C levels, LDL-C/HDL-C ratio, and increased concentrations of serum triglycerides, whereas no difference in these parameters was observed between TM and TI patients except for serum triglycerides that were found to be significantly higher in the TI group. The median values for plasma Lp-PLA2 were as follows: 874 (μg/l) (range, 752–953 µg/l), 636 µg/l (range, 564–768 µg/l), and 234 µg/l (range, 189–298 µg/l) for TM, TI children, and the controls, respectively. While the median values for serum TNF-α were as follows: 3.96 (ng/ml) (range, 3.69–4.84 ng/ml), 2.65 ng/ml (range, 1.63–3.5 ng/ml), and 1.67 ng/ml (range, 1–2.4 ng/ml) for TM, TI children, and the controls, respectively. The two studied pro-inflammatory markers were significantly higher in both patient groups compared with the control group with TM patients expressing higher levels of both markers compared with TI patients (P < 0.001) ().
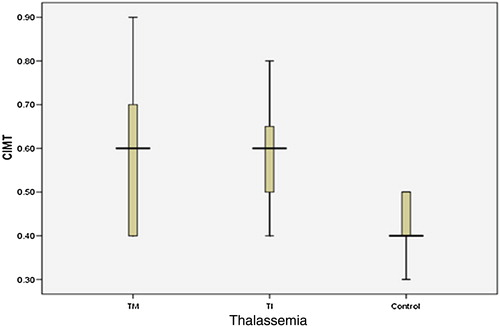
Regarding ultrasonographic evaluation, atherosclerotic plaque was not detected in any of our studied children. The mean values ± SD of cIMT were (0.58 ± 0.14, range 0.4–0.9 mm, 0.59 ± 0.12, range 0.4–0.8 mm, and 0.43 ± 0.08, range 0.3–0.5 mm) for TM, TI, and the control groups, respectively. The cIMT values of both patient groups were significantly increased compared with the control group ( and ).
For the controls, the 5th centile cIMT was 0.3 mm, the 50th centile was 0.4 mm, and the 95th centile was 0.5 mm. Elevated values of cIMT measurements were defined as value above the 95th centile of the healthy control subjectsCitation27 (>0.5 mm). According to this, among the total studied 42 thalassemia children, 24 patients (57%) had elevated cIMT values of >0.5 mm and 18 patients (43%) had non-elevated values (≤0.5 mm). Out of the patients with elevated cIMT, 12 had TM (12/22 = 54.5%) and 12 had TI (12/20 = 60%) and 12 out of these 24 children (50%) were below 12 years old. Thalassemia patients with elevated cIMT were significantly older with higher heart rate compared to those with non-elevated cIMT. The mean yearly serum ferritin, AST, and the pro-inflammatory markers plasma Lp-PLA2 and serum TNF-α were significantly higher in patients with elevated cIMT compared to those with non-elevated cIMT. No significant differences were found in terms of other tested parameters between these groups ().
Table 2. Comparison between thalassemia patients regarding cIMT categories
Univariate analysis was performed to study the correlation between cIMT and the tested variables (). Among all thalassemia patients and in both patient groups, cIMT values were positively correlated with serum ferritin over the last year, plasma Lp-PLA2 and serum TNF-α. Pre-transfusion Hb had significant negative correlation with cIMT in all studied thalassemia children. Within TM patients, cIMT had significant positive correlation with LDL-C/HDL-C ratio while within TI patients it was correlated with the age. Disease duration was not correlated with cIMT in either patient group or in the total thalassemia children. In the control group, a significant positive correlation was found between cIMT and the age, BMI, TC, LDL-C, LDL-C/HDL-C ratio, and plasma Lp-PLA2.
Table 3. Correlation between cIMT and variable parameters in the studied groups
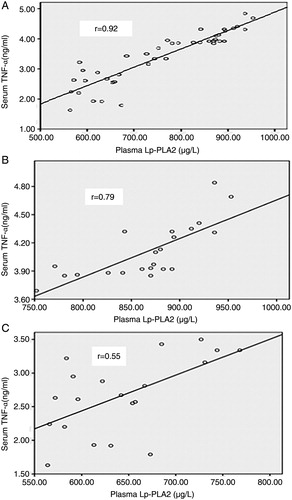
Plasma Lp-PLA2 and serum TNF-α had significant positive correlation with each other when tested in all thalassemia children (r = 0.92, P < 0.0001) and within each thalassemia group (r = 0.79, P < 0.0001 for TM and r = 0.55, P = 0.01 for TI) (A–C).
Figure 2. (A) Correlation between plasma Lp-PLA2 (μg/l) and serum TNF-α (ng/ml) in all studied thalassemia children (r = 0.92, P < 0.0001). (B) Correlation between plasma Lp-PLA2 (μg/l) and serum TNF-α (ng/ml) in TM children (r = 0.79, P < 0.0001). (C) Correlation between plasma Lp-PLA2 (μg/l) and serum TNF-α (ng/ml) in TI children (r = 0.55, P = 0.01).
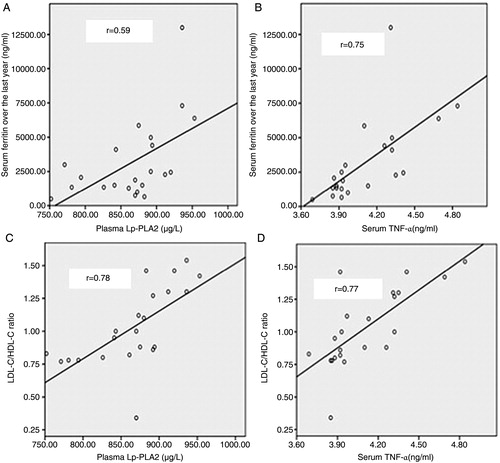
In the TM group, both plasma Lp-PLA2 and serum TNF-α had significant positive correlation with serum ferritin (r = 0.59, P = 0.004 for plasma Lp-PLA2 and r = 0.75, P < 0.0001 for serum TNF-α, respectively) (A and B). This was also true for LDL-C/HDL-C ratio (r = 0.78, P < 0.0001 for plasma Lp-PLA2 and r = 0.77, P < 0.0001 for serum TNF-α, respectively) (C and D). However, no significant correlation was found between plasma Lp-PLA2 or serum TNF-α with any of the other parameters of lipid profiles in either patient group.
Figure 3. (A) Correlation between plasma Lp-PLA2 (μg/l) and serum ferritin (ng/ml) in TM children (r = 0.59, P = 0.004). (B) Correlation between serum TNF-α (ng/ml) and serum ferritin (ng/ml) in TM children (r = 0.75, P < 0.0001). (C) Correlation between plasma Lp-PLA2 (μg/l) and LDL-C/HDL-C ratio in TM children (r = 0.78, P < 0.0001). (D) Correlation between serum TNF-α (ng/ml) and LDL-C/HDL-C ratio in TM children (r = 0.77, P < 0.0001).
Thalassemia children with HCV infection were of older age and had significantly higher BMI, ALT, AST, and cIMT compared to those without HCV infection. No significant difference was found between these subgroups regarding the parameters of lipid profile, levels of plasma Lp-PLA2 or serum TNF-α. Premature atherosclerosis had occurred more frequently in HCV-positive thalassemia children (14 out of 18) when compared to those without this infection (10 out of 24) (P = 0.019) ().
Table 4. The relation between HCV infection and premature atherosclerosis among thalassemia children
Discussion
It is worth mentioning that subclinical and premature atherosclerosis are not synonymous. While subclinical atherosclerosis is the asymptomatic phase of atherosclerosis,Citation28 atherosclerosis is defined as premature if it begins at or before the age of 49 years.Citation29
Considering cIMT as an excellent marker of subclinical atherosclerosis,Citation12 this non-invasive tool was applied to a group of cardiac-free thalassemia children not suffering from any vascular symptom in a study that involved the two disease categories (TM and TI) comparing to the healthy group. The significant increased cIMT in each patient groups compared to the controls provides a good evidence of the presence of premature atherosclerosis in vascular-free TM and TI patients.
To our knowledge, until now, there is no international consensus age distribution of cIMT in the healthy general pediatric population.Citation30 Jourdan et al.Citation31 provided cIMT range and distribution of healthy Caucasian adolescents aged 10–20 years with the highest 97th percentile value (0.48 mm) was reported for the 18 years old children. Recent study done by de Arriba Muñoz et al.Citation32 reported this distribution among Spanish children aged 4–15 years in terms of mean ± SD with the highest value of 0.5 mm was for those of 15 years (mean ± 2 SD). In accordance to this, cIMT 95th percentile in our control group was 0.5 mm. The majority of the studied thalassemia children (24 out of 42 = 57%) had elevated cIMT (>0.5 mm), a finding that supports the argument for the presence of premature atherosclerosis among our studied young thalassemia being more prevalent among TI children (60% of TI versus 54.5% of TM).
Recently, two studies reported premature atherosclerosis as an important complication among β-TM children.Citation19,Citation20 However so far, the presence of this vascular complication based on elevated cIMT had been documented in β-TI adults in an only one studyCitation17 without any data about this complication among β-TI children.
Limited data are available regarding the atherogenic risk of thalassemia, though it may be related to several contributors. It was found that nitric oxide bioavailability disruption by free Hb could trigger vascular dysfunction in thalassemia patients.Citation33 Moreover, unpaired Hb α-chains might enhance LDL oxidative modification rendering them more atherogenic.Citation34
For many years, dyslipidemia was emerging as a strong risk for atherosclerosis.Citation35 The previous studies provided discordant results about the lipid profile pattern of thalassemia patients. Some authors adduced atherogenic phenotypeCitation14,Citation36 while others reported anti-atherogenic lipid profiles among these patients.Citation37,Citation38 The results of this study were in favor of the anti-atherogenic side, where both patient groups exhibited significant lower serum TC, LDL-C, HDL-C levels, and LDL-C/HDL-C ratio compared with the control group. The only adverse atherogenic parameter was the significantly higher serum triglycerides found in both patient groups compared with the controls, being higher in TI compared with TM children. Similar results were recorded in Egyptian β-thalassemia children.Citation39 Gursel et al.Citation20 disclosed similar results among their studied TM children except for triglycerides where no significant difference was found between patient and control groups.
β-thalassemia has LDL-lowering effect that could be attributed to increased LDL-C uptake by the active erythroid progenitor cells providing a source of cholesterol together with enhanced inflammatory cytokines secretion that inhibit LDL-C hepatic secretion and stimulate its catabolism.Citation40 Actually, the interplay between macrophage monocyte activation together with hepatic dysfunction due to iron overload could explain the lipoprotein pattern observed in our study. On the other hand, hyper-triglyceridemia might be related to reduced extra-hepatic lipolytic activity.Citation38 Raised triglycerides provide deleterious atherogenic risk as they stimulate small LDL particles generation and lower HDL-C levels.Citation41 Hence, the higher prevalence of premature atherosclerosis in our TI patients could be explained by their significantly higher fasting triglycerides compared with TM children.
This observed lipid profile phenotype with characteristic low LDL-C playing in harmony with blood rheology may give an explication for the distinctive rarity of coronary artery disease with more susceptibility to non-coronary thromboembolism reported in thalassemia patients.Citation2
Studying the relation between lipid profiles and premature atherosclerosis, our results revealed that within the patient groups, LDL-C/HDL-C ratio was the only tested lipid profile parameter that was related to cIMT in the TM group.
All previous results reflected the importance of studying other biological contributors, particularly the inflammatory markers that could provide a suitable approach to study inflammation in early atherosclerosis. In our study, the inflammatory aspect of atherosclerosis has been elucidated by plasma Lp-PLA2 and serum TNF-α levels evaluation. The remarkable findings in this study were the significant elevation of these markers among both thalassemia groups compared with healthy children being higher in TM compared to TI children (P < 0.001).
It has been postulated that LDL-C level and its clearance rate from the circulation are the major plasma Lp-PLA2 levels determinants.Citation42 Taking into account that our thalassemia children had low LDL-C, it seems strange for them to exhibit high plasma Lp-PLA2 level. But, this could be interpreted on the basis of the fact that the majority of the LDL-associated Lp-PLA2 is bound to atherogenic small dense LDL particlesCitation43 which are abundant in milieu of hypertriglyceridemia.Citation44 Elevated plasma levels of Lp-PLA2 mass and activity being higher in TM compared with TI adults were reported in a single earlier study done by Tselepis et al.Citation16 who attributed this elevation to its enhanced monocytes/macrophages secretion played by pro-inflammatory oxPLs formed under conditions of increased oxidative stress of thalassemia.
Since many years, the association between Lp-PLA2 and cardiovascular disease (CVD) had been recognized.Citation45,Citation46 In this context, our results detected significant higher Lp-PLA2 plasma level in thalassemia children with elevated cIMT compared with its level in patients with non-elevated cIMT (P < 0.01). The enzyme level also displayed significant correlations with cIMT in both studied patient groups (P < 0.0001 for both), results that may give a proof for Lp-PLA2 participation in premature atherosclerosis in thalassemia children. These results also advocate the hypothesis suggested by Tselepis et al.Citation16 that high plasma Lp-PLA2 levels in milieu with a relatively anti-atherogenic cholesterol level may contribute to premature carotid atherosclerosis with subsequent stroke but not to coronary artery disease in cardiac disease-free β-thalassemia patients.
Despite its suggested role in CVD, Lp-PLA2 has an antioxidant activity. The possible role of this enzyme in atherogenesis and whether it dominantly exerts anti- or pro-atherogenic effect and whether its elevation is a cause or a result of the atherogenic process remain an un-answered question.Citation47 However, the pro-inflammatory pro-atherogenic role of Lp-PLA2 was the dominant in the majority of researches performed in the last years. This detrimental action is related to the lysophospholipids (generated during phospholipids hydrolysis) that are involved in atherosclerotic process through their influence on neutrophils function with its attractant effect for monocytes and macrophages enhancing the inflammatory process.Citation48
It was reported that the Lp-PLA2 activity in adolescents was positively associated with TC and LDL-C.Citation49 This relation phenotype was not found among our thalassemia children.
Nevertheless, among our studied TM children, Lp-PLA2 plasma level had strong significant positive linear relation with LDL-C/HDL-C ratio (P < 0.0001). This finding together with significant positive correlation between cIMT and this lipid profile parameter in this patient group could be considered as a strong pretext in favor of Lp-PLA2 pro-atherogenic effect putting in mind that, altogether with high triglycerides, high LDL-C/HDL-C ratio has been suggested to be associated with highest cardiovascular risk.Citation50
Parallel to plasma Lp-PLA2, serum TNF-α displayed the same results pattern among our studied thalassemia patients as these two markers were correlated with each other in all studied thalassemia patients (P < 0.0001) and in both patient groups (P < 0.0001 for TM and P = 0.01 for TI). This uniform relation could be related to the cytokines stimulatory effect of oxPLs hydrolysis products generated by Lp-PLA2.Citation51
High serum TNF-α concentrations had been documented among thalassemia patients regardless their splenic status. Authors assigned this rise to macrophage activation by iron overload, the antigenic stimulation promoted by chronic transfusion therapy and to accelerated erythropoiesis.Citation52,Citation53
Tselepis et al.Citation16 disclosed significant increase of serum TNF-α level in both the TM and TI patient groups compared with the controls being higher in TM, a finding that came in accordance with our results in this regards. On the contrary, Hahalis et al.Citation17 reported non-significant difference in serum TNF-α level between his studied TI adults and the healthy controls. It is notable that TNF-α is one of the cytokines involved in LDL-C-lowering effect in thalassemia patients.Citation40
This systemic inflammatory cytokine is involved in all stages of atherosclerosis from initiation to thrombotic events and plaque rupture induction through atheroma evolution.Citation54,Citation55 In favor of this notion, our study results revealed the presence of significant positive correlation between TNF-α serum level and cIMT in both patient groups. In addition, this cytokine serum level was significantly higher in patients with elevated cIMT compared with those having non-elevated cIMT values (P < 0.05). Among the TM group, TNF-α serum level had positive linear relation with the atherogenic LDL-C/HDL-C ratio. The link between high serum level of this mediator and atherosclerosis among thalassemia patients may be accounted for by the antigenic effect of free oxidized Hb in these patients which enhance specific T lymphocytes pro-inflammatory cytokines secretion including TNF-α.Citation56
Data about the role of this cytokine as an atherosclerosis mediator in thalassemia patients are few in previously published studies. While in one study performed upon TI adults, Hahalis et al.Citation17 disclosed non-significant correlation between TNF-α and cIMT, this mediator was one of the independent predictors of cIMT among TM patients in another study.Citation4
The significant higher level of plasma Lp-PLA2 and serum TNF-α in TM compared to TI children in our study could be explained by what was reported that TM patients usually present more severe anemia requiring frequent blood transfusions compared with TI that leads to extra iron, more inflammation, and oxidative stress.Citation5 But, this could be criticized by the finding of non-significant difference in serum ferritin between TM and TI children in this study. The difference in the two tested markers (Lp-PLA2 and TNF-α) between the two forms of thalassemia (TM and TI) could be related to other biological factors not tested in this work that might be new topics of extra researches.
Chronic HCV infection had been considered a risk factor for early carotid atherosclerosis.Citation57,Citation58
Consistent with this, premature atherosclerosis was significantly prevalent in our studied HCV-positive thalassemia children (18 out of 42) who exhibited significant higher mean cIMT as compared to HCV-negative patients (P < 0.05).
It was reported that HCV infection could influence Lp-PLA2 plasma activity.Citation59 HCV-infected patients showed a significant decrease of this enzyme activity that was only recovered by viral clearance after anti-viral treatment.Citation60 Against this, in this study HCV infection had no impact on Lp-PLA2 plasma level where no significant difference was found in its level between HCV positive and negative thalassemia children. The same was found by Tselepis et al.Citation16 who found that the differences in Lp-PLA2 levels among studied groups were not altered after exclusion of HCV-infected thalassemia patients from the statistical analysis. These contradictory results may be related to the difference in the study population, since the previous studies had been performed on non-thalassemic individuals.
As a potent anti-viral cytokine, circulating TNF-α level increases during HCV infection.Citation61,Citation62 Its production is induced in viral-infected hepatocytes.Citation63 In this regards, our results revealed non-significant difference in serum TNF-α level between HCV-positive and HCV-negative thalassemia children. This could be interpreted by what was reported that, elevated TNF-α level correlates with the severity of hepatic inflammation, fibrosis, and tissue injuryCitation64 putting in mind that the fact that all our studied HCV-positive thalassemia children had low viral load.
The inevitable thalassemia complication of iron overload had been linked to atherosclerosis.Citation15 Free iron has a deleterious effect related to its catalytic role in free radical reactions with LDL oxidative modification, the key step in atherosclerotic vascular events.Citation65 Contradictory results were traced in other studies regarding relation of serum ferritin to cIMT among thalassemia patients. Some reported significant positive correlationCitation14 while others revealed non-significant correlation between these two parameters.Citation17,Citation20 Lai et al.Citation36 considered iron load as a risk factor for atherosclerosis in β-TI patients. In this regard, cIMT was correlated with serum ferritin among all studied thalassemia children (P = 0.005) as well as among the TM group (P = 0.02) with borderline relation in the TI group (P = 0.049). Furthermore, thalassemia patients with elevated cIMT had significantly higher serum ferritin level compared with patients with non-elevated cIMT. At the same time, among TM children both plasma Lp-PLA2 and serum TNF-α were significantly correlated with serum ferritin (P = 0.004 for Lp-PLA2 and P < 0.0001 for TNF-α). Ferritin with its potent pro-oxidant effect was considered as a potential modulator of plasma Lp-PLA2 activity.Citation66 It is also a good inducer for TNF-α rise.Citation52,Citation53
All of the above evidence lends credence to the idea that pro-inflammatory state in thalassemia patients may predominate atheroprotective mechanisms of chelation regimen enhancing vascular injury and atherogenesis.Citation4 Considering that increased plasma Lp-PLA2 and serum TNF-α levels were reported to be associated with high incidence of stroke,Citation67,Citation68 this study results may add an explanation for the predominance of non-coronary over the coronary vascular complications among thalassemia patients reported in previous studies.
In conclusion, this study demonstrated the presence of premature subclinical carotid atherosclerosis in thalassemia children despite their relative anti-atherogenic lipid profile being more prevalent among the TI group. This refers to the suitability of these patients to this vascular complication starting at an early age. Likewise, the eminent observations of our study were the elevated plasma Lp-PLA2 and serum TNF-α levels among the studied thalassemia children which had good correlation with cIMT and serum ferritin providing a pertinent relationship between thalassemia and premature atherosclerosis with a robust link between this complication and the inflammatory mediators (Lp-PLA2 and TNF-α), a suggestion that needs study on large population to document cause–effect relationship.
Disclaimer statements
Contributors SMR is responsible for the study concept and design, overseeing data collection, data interpretation, literature search, drafting the manuscript, figures, and tables, and writing/revising the manuscript. MAS is responsible for performing the biochemical analysis of the studied parameters and sharing in writing/revising the manuscript. OMO is responsible for ultrasonographic measuring of cIMT. ASS is responsible for overseeing data collection.
Funding Personal.
Conflicts of interest None.
Ethics approval Ethical clearance from the ethical committee in our medical school (Menoufia Faculty of medicine, Egypt) was obtained before study began.
References
- Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353(11):1135–46.
- Taher A, Isma'eel H, Mehio G, Bignamini D, Kattamis A, Rachmilewitz EA, et al. Prevalence of thromboembolic events among 8860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost. 2006;96(4):488–91.
- Cheung YF, Chan GC, Ha SY. Arterial stiffness and endothelial function in patients with β-thalassemia major. Circulation 2002;106(20):2561–6.
- Hahalis G, Kremastinos DT, Terzis G, Kalogeropoulos AP, Chrysanthopoulou A, Karakantza M, et al. Global vasomotor dysfunction and accelerated vascular aging in beta-thalassemia major. Atherosclerosis 2008;198(2):448–57.
- Taher A, Isma'eel H, Cappellini MD. Thalassemia intermedia; revisited. Blood Cells Mol Dis. 2006;37(1):12–20.
- Kyriakou DS, Alexandrakis MG, Kyriakou ES, Liapi D, Kourelis TV, Passam F, et al. Activated peripheral blood and endothelial cells in thalassemia patients. Ann Hematol. 2001;80(10):577–83.
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473(7347):317–25.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95.
- Tselepis AD, John Chapman M. Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atheroscler Suppl. 2002;3(4):57–68.
- Tellis CC, Tselepis AD. The role of lipoprotein-associated phospholipase A2 in atherosclerosis may depend on its lipoprotein carrier in plasma. Biochim Biophys Acta. 2009;1791(5):327–38.
- Girn HR, Orsi NM, Homer-Vanniasinkam S. An overview of cytokine interactions in atherosclerosis and implications for peripheral arterial disease. Vasc Med. 2007;12(4):299–309.
- Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006;37(1):87–92.
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115(4):459–67.
- Tantawy AA, Adly AA, El Maaty MG, Amin SA. Subclinical atherosclerosis in young beta-thalassemia major patients. Hemoglobin 2009;33(6):463–74.
- Akhlaghpoor S, Hoseini M, Jafarisepehr A. Association of iron overload based quantitative T2* MRI technique and carotid intima-media thickness in patients with beta-thalassemia: a cross-sectional study. BMC Cardiovasc Disord. 2010;10:62.
- Tselepis AD, Hahalis G, Tellis CC, Papavasiliou EC, Mylona PT, Kourakli A, et al. Plasma levels of lipoprotein-associated phospholipase A(2) are increased in patients with β-thalassemia. J Lipid Res. 2010;51(11):3331–41.
- Hahalis G, Kalogeropoulos A, Terzis G, Tselepis AD, Kourakli A, Mylona P, et al. Premature atherosclerosis in non-transfusion-dependent β-thalassemia intermedia. Cardiology. 2011;118(3):159–63.
- Cheung YF, Chow PC, Chan GC, Ha SY. Carotid intima-media thickness is increased and related to arterial stiffening in patients with beta-thalassaemia major. Br J Haematol. 2006;135(5):732–4.
- Dogan M, Citak EC. The evaluation of carotid intima-media thickness in children with beta-thalassaemia major. Cardiol Young 2012;22(1):79–83.
- Gursel O, Kurekci AE, Tascilar E, Ileri T, Altun D, Tapan S, et al. Premature atherosclerosis in children with β-thalassemia major. J Pediatr Hematol Oncol. 2012;34(8):630–4.
- Thalassaemia International Federation. Guidelines for the cinical management of thalassaemia. 2nd revised edition. Nicosia, Cyprus: Thalassaemia International Federation; 2008 [accessed 2011 Jun 21]. Available from: http://www.thalassaemia.org.cy/pdf/Guidelines_2nd_revised_edition_EN.pdf.
- Mellouli F, El Borgi W, Kaabi H, Ben Hassen E, Sassi R, Hmida H, et al. [HFE gene mutations in Tunisian major beta-thalassemia and iron overload]. Transfus Clin Biol. 2006;13(6):353–7.
- Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19(12):1350–6.
- Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28(10):2077–80.
- Lippi U, Graziani MS, Schinella M, Manzato F, Bazzani R. Determination of high density lipoprotein cholesterol in venous and capillary whole blood. J Lipid Res. 1988;29(1):112–5.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
- Margeirsdottir HD, Stensaeth KH, Larsen JR, Brunborg C, Dahl-Jørgensen K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care 2010;33(9):2043–8.
- Toth PP. Subclinical atherosclerosis: what it is, what it means and what we can do about it. Int J Clin Pract. 2008;62(8):1246–54.
- Hansen ME, Valentine RJ, McIntire DD, Myers SI, Chervu A, Clagett GP. Age-related differences in the distribution of peripheral atherosclerosis: when is atherosclerosis truly premature? Surgery 1995;118(5):834–9.
- Böhm B, Hartmann K, Buck M, Oberhoffer R. Sex differences of carotid intima-media thickness in healthy children and adolescents. Atherosclerosis 2009;206(2):458–63.
- Jourdan C, Wühl E, Litwin M, Fahr K, Trelewicz J, Jobs K, et al. Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens. 2005;23(9):1707–15.
- de Arriba Muñoz A, Domínguez Cajal MM, Labarta Aizpún JI, Domínguez Cunchillos M, Mayayo Dehesa E, Ferrández Longás Á. [Carotid intima-media thickness; normal values from 4 years]. Nutr Hosp. 2013;28(4):1171–6.
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO III, Schechter AN, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–9.
- Altamentova SM, Marva E, Shaklai N. Oxidative interaction of unpaired hemoglobin chains with lipids and proteins: a key for modified serum lipoproteins in thalassemia. Arch Biochem Biophys. 1997;345(1):39–46.
- Shamir R, Kassis H, Kaplan M, Naveh T, Shehadeh N. Glycemic control in adolescents with type 1 diabetes mellitus improves lipid serum levels and oxidative stress. Pediatr Diabetes 2008;9(2):104–9.
- Lai ME, Vacquer S, Carta MP, Spiga A, Cocco P, Angius F, et al. Thalassemia intermedia is associated with a proatherogenic biochemical phenotype. Blood Cells Mol Dis. 2011;46(4):294–9.
- Hartman C, Tamary H, Tamir A, Shabad E, Levine C, Koren A, et al. Hypocholesterolemia in children and adolescents with beta-thalassemia intermedia. J Pediatr. 2002;141(4):543–7.
- Chrysohoou C, Panagiotakos DB, Pitsavos C, Kosma K, Barbetseas J, Karagiorga M, et al. Distribution of serum lipids and lipoproteins in patients with beta thalassaemia major; an epidemiological study in young adults from Greece. Lipids Health Dis. 2004;3:3–8.
- Nasr MR, Abdelmaksoud AM, Abd El-Aal KS, Mabrouk NA, Ismael WM. Plasma lipid profile and lipid peroxidation in beta-thalassemic children. J Clin Lipidol. 2008;2(6):405–9.
- Calandra S, Bertolini S, Pes GM, Deiana L, Tarugi P, Pisciotta L, et al. Beta-thalassemia is a modifying factor of the clinical expression of familial hypercholesterolemia. Semin Vasc Med. 2004;4(3):271–8.
- Grundy SM, Vega GL. Two different views of the relationship of hypertriglyceridemia to coronary heart disease: implications for treatment. Arch Intern Med. 1992;152(1):28–34.
- Tsimihodimos V, Karabina SA, Tambaki AP, Bairaktari E, Miltiadous G, Goudevenos JA, et al. Altered distribution of platelet activating factor-acetylhydrolase activity between LDL and HDL as a function of the severity of hypercholesterolemia. J. Lipid Res. 2002;43(2):256–63.
- Tselepis AD, Dentan C, Karabina SA, Chapman MJ, Ninio E. PAF-degrading acetylhydrolase is preferentially associated with dense LDL and VHDL-1 in human plasma. Catalytic characteristics and relation to the monocyte-derived enzyme. Arterioscler Thromb Vasc Biol. 1995;15(10):1764–73.
- Chapman MJ. Metabolic syndrome and type 2 diabetes: lipid and physiological consequences. Diab Vasc Dis Res. 2007;4 (Suppl 3):S5–8.
- Rosenson RS, Stafforini DM. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J Lipid Res. 2012;53(9):1767–82.
- Persson M, Nilsson J, Nelson J, Hedblad B, Berglund G. The epidemiology of Lp-PLA2: distribution and correlation with cardiovascular risk factors in a population-based cohort. Atherosclerosis 2007;190(2):388–96.
- McConnell JP, Hoefner DM. Lipoprotein-associated phospholipase A2. Clin Lab Med. 2006;26(3):679–7, vii.
- Frasch SC, Zemski-Berry K, Murphy RC, Borregaard N, Henson PM, Bratton DL. Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J Immunol. 2007;178(10):6540–8.
- ; The Lp-PLA2 Studies CollaborationThompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, et al. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375(9725):1536–44.
- Grundy SM. Small LDL, atherogenic dyslipidemia, and the metabolic syndrome. Circulation 1997;95(1):1–4.
- Silva IT, Mello AP, Damasceno NR. Antioxidant and inflammatory aspects of lipoprotein-associated phospholipase A2 (Lp-PLA2): a review. Lipids Health Dis. 2011;10:170.
- Butthep P, Rummavas S, Wisedpanichkij R, Jindadamrongwech S, Fucharoen S, Bunyaratvej A. Increased circulating activated endothelial cells, vascular endothelial growth factor, and tumor necrosis factor in thalassemia. Am J Hematol. 2002;70(2):100–6.
- Gharagozloo M, Karimi M, Amirghofran Z. Double-faced cell-mediated immunity in β-thalassemia major: stimulated phenotype versus suppressed activity. Ann Hematol. 2009;88(1):21–7.
- Vaddi K, Nicolini FA, Mehta P, Mehta JL. Increased secretion of tumour necrosis factor-alpha and interferon-gamma by mononuclear leukocytes in patients with ischaemic heart disease. Relevance in superoxide anion generation. Circulation 1994;90(2):694–9.
- Ross R. Atherosclerosis – an inflammatory disease. New Engl J Med. 1999;340(2):115–26.
- Profumo E, Buttari B, Riganò R. Oxidized haemoglobin as antigenic target of cell-mediated immune reactions in patients with carotid atherosclerosis. Autoimmun Rev. 2009;8(7):558–62.
- Boddi M, Abbate R, Chellini B, Giusti B, Solazzo V, Soft F, et al. HCV infection facilitates asymptomatic carotid atherosclerosis: preliminary report of HCV RNA localization in human carotid plaques. Dig Liver Dis. 2007;39 (Suppl 1):S55–60.
- Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, et al. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis 2012;221(2):496–502.
- Caini P, Guerra CT, Giannini C, Giannelli F, Gragnani L, Petrarca A, et al. Modifications of plasma platelet-activating factor (PAF)-acetylhydrolase/PAF system activity in patients with chronic hepatitis C virus infection. J Viral Hepat. 2007;14(1):22–8.
- Guerra CT, Caini P, Giannini C, Giannelli F, Gragnani L, Petrarca A, et al. Effect of chronic hepatitis C virus infection on inflammatory lipid mediators. Dig Liver Dis. 2007;39 (Suppl 1):S76–82.
- Antonelli A, Ferri C, Ferrari SM, Marchi S, De Bortoli N, Sansonno D, et al. N-terminal pro-brain natriuretic peptide and tumor necrosis factor-alpha both are increased in patients with Hepatitis C. J Interferon Cytokine Res. 2010;30(5):359–63.
- Sayed-Ahmed L, Kotb N, El-Serogy H, El-Shazly S, Eid M. TNF-alpha and CXCL-10 correlation with insulin resistance in patients with chronic hepatitis C virus infection. Egypt J Immunol. 2010;17(1):101–11.
- González-Amaro R, García-Monzón C, García-Buey L, Moreno-Otero R, Alonso JL, Yagüe E, et al. Induction of tumor necrosis factor alpha production by human hepatocytes in chronic viral hepatitis. J Exp Med. 1994;179(3):841–8.
- Falasca K, Ucciferri C, Dalessandro M, Zingariello P, Mancino P, Petrarca C, et al. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann Clin Lab Sci. 2006;36(2):144–50.
- Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Toxicol Appl Pharmacol. 2005;202(2):199–211.
- Tsimikas S, Willeit J, Knoflach M, Mayr M, Egger G, Notdurfter M, et al. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. Eur Heart J. 2009;30(1):107–15.
- Gorelick PB. Lipoprotein-associated phospholipase A2 and risk of stroke. Am J Cardiol. 2008;101(12A):34F–40F.
- Cure MC, Tufekci A, Cure E, Kirbas S, Ogullar S, Kirbas A, et al. Low-density lipoprotein sub-fraction, carotid artery intima-media thickness, nitric oxide, and tumor necrosis factor alpha are associated with newly diagnosed ischemic stroke. Ann Indian Acad Neurol. 2013;16(4):498–503.