Abstract
Objectives
Bone marrow (BM) aspiration and trephine biopsy is one of the most valuable procedures in the evaluation of hematological disorders. There is a shortage of published literature regarding the indications, procedure, and outcome of bone marrow examination (BME) in neonates and infants. The aim of the present study is to analyze the common indications of performing BME and to assess the spectrum of disorders diagnosed from BM of neonates and infants.
Methods
A retrospective analysis of BMEs performed in infants over a period of 5 years, between 2009 and 2013 was done.
Results and discussion
A total of 297 BME were performed on 285 infants, which constitutes 10.3% of pediatric BME procedures during the same period. In our institute, BME is routinely performed by trained pathologists from posterior superior iliac spine in children including infants and neonates with an overall sample adequacy of 97%. Evaluation of cytopenias and suspicion of storage disorder were the most common indications for BME procedure, while acute leukemias and storage disorders were the most common diagnoses offered in infant BM.
Conclusions
Posterior superior iliac spine is a good site of BME in neonates and infants. BM trephine biopsy is a difficult procedure in this age group, however remains indispensable in situations where an infiltrative pathology is suspected. BME not only helps to make specific diagnoses but should also be used as an extremely valuable, quick, and economically viable procedure to exclude major hematological disorders including certain forms of storage disorder and hematological malignancy in this age group.
Introduction
Bone marrow examination (BME), which includes aspiration and trephine biopsy, is one of the most valuable procedures in the evaluation of hematological disorders. These are invasive procedures performed by trained individuals using standard techniques and allow cytologic, immunophenotypic, cytogenetic, molecular assessment, and other specialized investigations. It is done with great care in infants and neonates with definite clinical indications like cytopenias of one or more lineages, the presence of immature or atypical cells in peripheral blood film (PBF), suspicion of hematological malignancies or storage disorders, and staging of lymphoreticular as well as certain non-hematological malignancies. There is a shortage of published literature regarding the indications, procedure, and outcome of BME in infants. The aim of the present study is to analyze the common indications of performing BME and to assess the spectrum of disorders diagnosed from infant bone marrow (BM).
Materials and methods
This was a retrospective study, which analyzed patient records from archives of Department of Hematology of tertiary care institute of north India. The requests and reports of all BMEs performed in infants and neonates (≤1year of age) over a period of 5 years, between January 2009 and December 2013 were retrieved from the archives. They were systematically analyzed for demographic profile of patients, indications for performing the investigation, hemogram findings, adequacy of the procedure, and the diagnoses offered by experienced hematopathologists of the institute. The indications and diagnoses of BMEs were grouped in appropriate categories for comparison.
BME was performed after informed consent by standard techniquesCitation1,Citation2 using disposable needles. All procedures in the institute were carried out by trained hematopathologists. Posterior superior iliac spine (PSIS) was the preferred site of the procedure and was done under short general anesthesia. A bone marrow aspirate (BMA) was performed in all cases, and bone marrow trephine biopsy (BMTB) was performed whenever indicated. BMA and unilateral BMTB were performed in suspected cases of acute leukemias, where as bilateral BMTB were performed in cases of suspected BM failure/evaluation of cytopenias, hemophagocytic lymphohistiocytosis (HLH), storage disorders, fever of unknown origin (FUO), and staging non-hematological malignancies. Samples for immunophenotyping, molecular investigations, and cytogenetics were routinely collected during the procedure; however, the results of these procedures were not analyzed for the present study.
Results
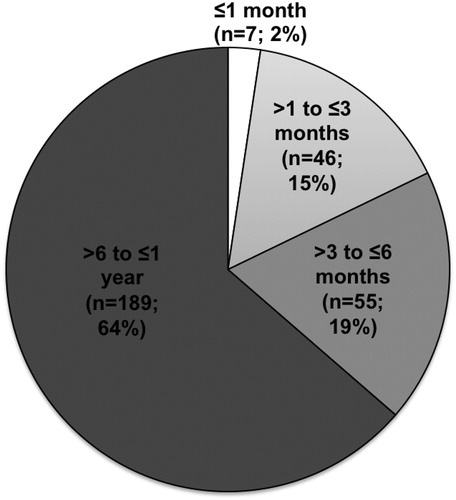
A total of 297 BMEs were performed on 285 infants accounting for 10.3% of a total of 2887 pediatric BMEs over a period of 5 years between 2009 and 2013. The age of children at the time of BME ranged from 17 days to 1 year with a median age of 9 months. Out of total of 297 BMEs, 7 (2.4%) were carried out on neonates (≤1 months), 46 (15.5%) were performed in children of age 1–3 months, 55 (18.5%) in children of age 3–6 months, and 189 (64%) procedures were performed in children between 6 months and 1 year (). There were 225 boys and 72 girls with a male female ratio of 3.1:1.
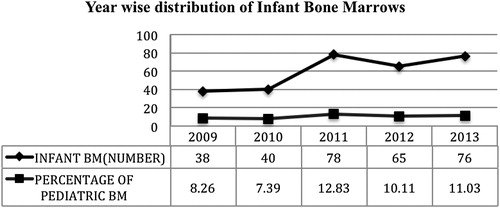
The number of BME procedures performed on infants increased in number from 38 in 2009 to 76 in 2013. The percentage of infant BM procedures out of all pediatric BM procedures, however, showed only marginal increase from 8.26 to 11.03% over 5 years ().
Adequacy of the procedure
All 297 BME procedures were carried out from PSIS. BMA was attempted in all cases and had BM particles in 213 (71.7%) cases. BMA was accompanied with a BMTB in 281 (94.6%) of procedures. BMA was suitable for evaluation irrespective of the presence of BM particles in 94%, while the BMTB was adequate for opinion in 88% of cases. Overall, 289 (97%) cases had sample (aspirate and/or trephine biopsy) adequate for diagnostic evaluation. On 26 occasions, BMA was adequate for opinion while BMTB was not, and the reverse happened on 6 occasions.
Indications
The BME was performed either for making a primary diagnosis (238 of 297; 80%), or as a part of staging in other malignancies (50 of 297; 16.8%) or for the evaluation of remission of malignancies involving BM (9 of 297; 3%). summarizes the indications for which 297 BME procedures were done. These indications were grouped in eight categories based on the most probable differential diagnosis considered after clinical evaluation of the patients.
Table 1. Indications for BME (N = 297)
The most frequent indication of BME in infants was evaluation of cytopenias, accounting to 82 (27.6%) procedures (). The patients evaluated for cytopenias included infants with unilineage, bilineage, or trilineage cytopenias with or without organomegaly. In this group, isolated anemia was present at the time of BME in 26 cases (31.7%), thrombocytopenia alone was present in 7 (8.5%) cases, and isolated leukopenia in 1 case. Bicytopenia was present in 42 cases (51.2%) and pancytopenia was present in 6 cases (7.3%). Second frequent indication for BME was suspected storage disorder/metabolic disorder on 66 (22.2%) occasions based on various clinical features, which includes dysmorphism, organomegaly, and abnormalities in developmental milestones. A BME was performed to diagnose or exclude hematological malignancy in 54 (18.2%) children. A clinical suspicion of a primary or secondary HLH was the chief indication of performing a BME on 33 (11.1%) occasions, when the child had variable degrees of cytopenia in the background of fever, organomegaly, altered liver function tests, and other biochemical parameters like serum ferritin, fibrinogen, and triglyceride levels. A staging BME was performed in 50 (16.8%) children, of whom 27 infants had a primary diagnosis of neuroblastoma (54%). Other indications included 12 cases of Langerhan cell histiocytosis (LCH), 2 cases each of retinoblastoma, germ cell tumor, non-Hodgkin lymphoma (NHL), and rhabdomyosarcoma, followed by 1 case each of hepatoblastoma, primitive neuroectodermal tumor, and malignant small round cell tumor (MSRCT), unclassified. A BME was performed on nine occasions for the evaluation of hematological remission following chemotherapy; seven of these were done following treatment for acute leukemia and one each after treatment for LCH and neuroblastoma involving BM.
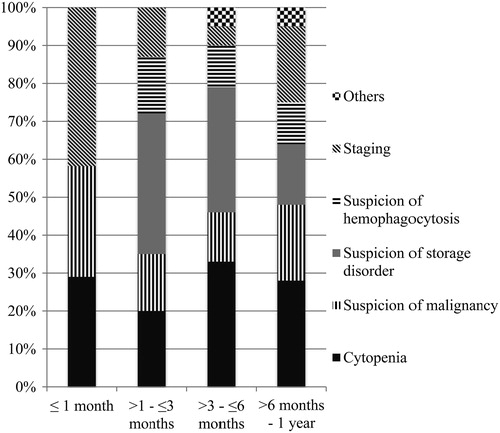
The indications of BME in various age groups are summarized in . Evaluation of cytopenias or a clinical suspicion of storage disorder was the most common indications for performing a BME in the post-neonatal period.
Diagnostic categories
Two-hundred and thirty-eight (80%) BM procedures were carried out as a primary diagnostic investigation. The procedure yielded sufficient material for a definite opinion in 231 (97%) cases. The diagnoses offered based on morphological assessment of BM samples were categorized in 18 groups and summarized in .
Table 2. Diagnoses offered on morphological evaluation of BM samples
Acute leukemia was diagnosed in 27 of 285 (9.5%) infants evaluated by BME. Out of this, 9 children had acute myeloid leukemia (AML) (acute megakaryoblastic leukemia (n = 3), acute myelomonocytic leukemia (n = 3), AML with maturation (n = 2), acute monocytic leukemia (n = 1)) and 18 children had B-lineage acute lymphoblastic leukemia (ALL), confirmed by flow cytometric immunophenotyping or immunohistochemistry. The PBF and BM findings were consistent with juvenile myelomonocytic leukemia (JMML) in five infants.
BM showed features, which were consistent with a storage disorder in 19 infants (6.7%). Storage cells showed a ‘crumbled tissue paper’ appearance morphologically consistent with Gaucher's disease in six of them. Thirteen infants had cells with ‘multi-vacuolated cytoplasm’ that can be seen in various lysosomal storage disorders like Niemann–Pick disease, Tay–Sachs disease, Tangier disease, etc. and need further confirmation by enzyme and/or molecular studies.
Sixteen infants with thrombocytopenia had either adequate or increased megakaryopoiesis on BME consistent with a diagnosis of ‘megakaryocytic thrombocytopenia’ while three infants had reduced megakaryopoiesis, one of which was a clinically suspected case of thrombocytopenia and absent corpus callosum syndrome. The term ‘megakaryocytic thrombocytopenia’ is used in situations where the thrombocytopenia is accompanied by normal or increased megakaryopoiesis in BM.
Hemophagocytosis was the significant finding in BM of 13 infants, all of whom had variable biochemical alterations and cytopenias (pancytopenia in 3, bicytopenia in 8, isolated anemia or thrombocytopenia in 1 child each).
A hypocellular BM favoring a constitutional or an acquired BM failure syndrome was present in eight infants. Two other infants had PBF and BM findings consistent for Pearson marrow syndrome, in the form of vacuoles in the erythroid and myeloid series of cells, and the presence of ring sideroblasts.
Eight infants had refractory anemia with variable degrees of reticulocytopenia and erythroblastopenia. Three of these children had severe erythroblastopenia satisfying the criteria for pure red cell aplasia (PRCA). Erythroid hyperplasia with significant dyserythropoiesis favoring a congenital dyserythropoietic anemia (CDA) was present in four BM samples, which were from three patients with transfusion-dependent anemia. Three BM samples from two infants who underwent BME for cytopenia and hepatosplenomegaly had myelofibrosis, while two other patients with cytopenia, showed necrosis of unknown etiology. The cause of myelofibrosis remained unclear during short follow-up in our institute.
Out of 238 occasions where a BME was performed for making a primary diagnosis, a specific diagnosis could not be offered in 116 cases (42%). In 86 of 116 (74%) cases, BME was performed with a clinical suspicion of storage disorder/HLH/hematological malignancy, however showed non-specific changes and did not reveal any storage cells, hemophagocytosis, or malignancy. Twenty-eight out of these 116 BMEs (25%) were performed for the evaluation of cytopenia with or without organomegaly. Megaloblastosis was seen in five of these cases and granulocyte hyperplasia with maturation arrest was seen in two cases. Twenty-one cases did not show any change, which could explain the cause of cytopenia. One case, where BME was performed for the evaluation of FUO, did not show organisms or granulomas or any other specific pathology. Another case, where the BME was performed for the evaluation of immunodeficiency also did not show any specific changes.
A BME was performed in 50 cases for the purpose of staging of a malignancy, of which 34 cases did not show BM involvement, 15 had evidence of infiltration, and 1 was inadequate for opinion. There were 34 BM procedures carried out for staging a tumor including neuroblastoma, retinoblastoma, primitive neuroectodermal tumor, hepatoblastoma, and rhabdomyosarcoma. Eight out of 27 patients (29.6%) with neuroblastoma and 1 case with rhabdomyosarcoma had involvement of BM, while none of the other MSRCT showed involvement. Five out of 12 patients (41.6%) with LCH had involvement of BM. One patient with nodal anaplastic large cell lymphoma (ALCL) showed involvement, while another case of lymphoblastic lymphoma did not involve the BM. Two cases of germ cell tumors also did not show BM involvement.
Out of 238 procedures where the BME was performed as a primary diagnostic investigation, we compared the number of cases where a definite morphological diagnosis could be offered against various indications. A definite morphological diagnosis could be offered in 51 of 82 (62.2%) BMEs performed for the evaluation of cytopenia, 35 of 54 (64.8%) infants with a clinical suspicion of hematological malignancy, 22 of 66 (33.3%) infants with suspected storage disorder, and only 6 of 33 (18%) infants with suspicion of primary or secondary HLH syndrome. Of the major indications, the diagnostic yield was maximum in conditions where the procedure was done for the evaluation of suspected hematological malignancy and cytopenias. It was of least diagnostic yield in situations where HLH was suspected.
BMTB accompanied BMA on 281 occasions, of which additional information was obtained from BMTB on at least 21 cases (7%). Myelofibrosis (n = 3), fibrosis associated with acute leukemia (n = 3), and BM necrosis (n = 2) could be seen only on BMTB. Storage cells (n = 5), LCH (n = 2), MSRCT (n = 2), and ALCL cells (n = 1) were either absent or were only occasionally seen in BMA, but were prominent in corresponding BMTB. The hypocellular nature of the BM was appreciable only in BMTB in three patients. BMA was useful, while the corresponding BMTB was not adequate for opinion in 26 cases while the reverse happened only on six occasions. A bilateral BMTB was performed on 192 cases. The pathological process affected only one of the sides in five patients; three cases showed infiltration by MSRCT. A case of LCH and another case of ALCL also showed unilateral infiltration of BM.
Discussion
Infantile BMEs constitute 10.3% (297 of 2887) of all the BMEs performed in pediatric population in our institute, whereas neonatal BM procedures accounted for only 0.2% (7 of 2887).
Technique of the procedure and adequacy of BME
BMA and BMTB can be performed from PSIS, anterior superior iliac crest, or anteromedial face of tibia. Though some people prefer tibia in children younger than 18 months, this site may fail to yield adequate material and there is a risk of fracture. In addition, it is difficult to perform biopsy from this site.Citation3 A technique using 19 gauge, half-inch Osgood needle to obtain marrow samples from tibia is also described.Citation4 In our institute, PSIS is the preferred site in children including infants and new born, which is well accepted.Citation2,Citation3
In adults, a BMTB is considered adequate if it measures 2–3 cm after processing and contains at least five to six intertrabecular spacesCitation5,Citation6 while some considers 1.5 or 1.6 cm as adequate for evaluation;Citation1,Citation7 both of which may be difficult to achieve in children. In one of the studies, BM trephine cores with <0.5 cm of BM after processing was considered inadequate for opinion in children evaluated for infiltration by neuroblastoma which is roughly equal to 0.75 cm before processing.Citation8 These are arbitrary criteria of adequacy and are not universally accepted. Hence in present study, adequacy was decided on a case-to-case basis depending on whether the biopsy provides adequate information or not. In our institute, unlike many health centers world over, trained pathologists, rather than clinicians routinely perform BME. In our experience, the BMA was suitable for evaluation in 94%, while the trephine biopsy was adequate in 88% of cases, making overall BME adequate in 97% of cases. The above figures definitely support PSIS to be a good site for carrying out BME.
Indications of BM examination in infants
This study revealed increase in number of infantile BME procedures over 5 years, however reflects the overall increase in the number of BME procedures performed during the same period. The most common indications for BME during neonatal period, early post-neonatal period (1–3 months), and after 6 months were ‘staging’, suspicion of a storage disorder, and evaluation of cytopenias, respectively. Overall, three most common indications for performing a BME in infants were evaluation of cytopenias, suspicion of a storage disorder, or hematological malignancy, together constituting 68% of all BMEs performed in this age group. Other major indications were staging in various non-hematopoietic neoplasms and suspicion of HLH syndrome.
Evaluation of cytopenia per se was the most common indication of BME accounting for 27.6% of the cases. A bicytopenia or pancytopenia was present in 59% of these patients. Isolated anemia was present in 31.7% and isolated thrombocytopenia in 8.5% of the infants who presented with cytopenias. A BME is performed in situations when the cause could not be determined by routine hematological investigations or when a specific BM pathology is suspected like malignancies, congenital or acquired dyserythropoietic anemias, congenital or acquired BM failure affecting one or more hematopoietic lineages, or if the cytopenias remained refractory to treatment. BME examination is performed in the evaluation of anemia in infants often after the exclusion of nutritional causes, hemolytic anemia, thalassemias, and hemoglobinopathies. It is also performed in situations, where there is a suspicion of an infiltrative marrow disorder or myelofibrosis.Citation9 A BME is definitely required for the diagnosis of sideroblastic anemia, PRCA, and CDA. BME used to be performed for the evaluation of thrombocytopenia, because of fear of missing acute leukemia or aplastic anemia. Large studies have shown that patients with isolated thrombocytopenia without anemia or absolute neutropenia or hepatosplenomegaly are unlikely to have a diagnosis of acute leukemia or aplastic anemia.Citation10 Currently, it is not an essential investigation in children with typical features of immune thrombocytopenia.Citation11
A clinical suspicion of storage disorder was the next common indication for the BME in 22.2% of the infants. The relatively inexpensive nature of the procedure prompts hematologists to use it as a screening tool in our setting, which may narrow down the spectrum of enzyme studies or genetic analysis necessary for a definite diagnosis. However, the BME is considered as a misuse in the evaluation of Gaucher's disease by some authors.Citation12
A suspicion of hematological malignancy was another major indication of BME in 18.1%. The procedure is sometimes avoided in cases with sufficient number of blasts in the periphery, which allows a definite diagnosis of acute leukemia based on morphology and immunophenotyping from peripheral blood. It is performed in all patients who have only few blasts in the periphery as well as for the subclassification of AML, which needs evaluation of BM cytology. The chance of successful cytogenetics is higher with BM samples. It allows establishing a baseline for further comparison with BMA performed after treatment. The assessment of trilineage dysplasia is better in BM samples.Citation2
A clinical diagnosis of primary or secondary HLH was the indication for BME in 11% of infants who presented with fever, splenomegaly, and cytopenias. BME is performed to look for the evidence of hemophagocytosis, and helps to rule out other diseases which may mimic HLH clinically.Citation13 Similarly, BME is an inexpensive ancillary investigation useful in establishing the diagnosis of FUO in appropriate clinical settings.Citation14
The indication of BME was staging in 16.8% of the infants with more than half of them having a primary diagnosis of neuroblastoma. BMA and BMTB are the standard procedures in the staging of malignant round cell tumorsCitation1, and involvement of BM indicates stage 4 or 4S according to International Neuroblastoma Staging System.Citation15 If BM is involved during initial staging marrow, the procedure is repeated during follow-up to assess response to treatment.
Diagnostic categories
The most common pathology diagnosed from infantile BM in our study was acute leukemia (11.34% of infants who underwent the procedure as a primary diagnostic investigation) and infantile leukemias constituted 1% (27 of 2887) of all pediatric BMs. This is an underestimate of the true frequency as we have not included those cases in which BME was not performed and were diagnosed from PBF alone. Infantile leukemias are rare but second commonest malignancy in infantile age group with an approximate incidence of 41 cases per million in the USA. ALL is slightly commoner than AML in a ratio of 1.3:1 and it shows an increased risk of occurrence in females in this age group. A high proportion of these cases are known in the literature to show translocation involving MLL gene on chromosome 11q23.Citation16 In our study, ALL was nearly twice as common as AML and showed male preponderance (1.5:1). There was only a single case of neonatal leukemia in our study, which was a case of Down syndrome-associated AML. Neonatal leukemias are the second common malignancy in this age group, however are rare with an incidence of one to five cases per million live births. They are believed to develop in utero and could be transient or non-transient. AML is more common accounting for two-third of the acute leukemias and the most common subtypes are French American British (FAB) M5, M4, and M7. MLL rearrangement is reported in up to 40% of cases of neonatal leukemias.Citation17
The PBF and BM findings were consistent with JMML in 2% (n = 5) of all infantile BMs performed as a primary diagnostic investigation. JMML accounts for 2–3% of all childhood cancers. The diagnosis requires examination of PBF, supplemented by ancillary investigations including cytogenetic evaluation. A BME is often performed to exclude acute myelomonocytic leukemia.Citation18,Citation19 It can occur from 1 month to 14 years of age and boys are affected twice as frequently as girls.Citation18 In our study, all infants with JMML were boys and the youngest child was of 3 months age.
The second common diagnosis in infantile BM was storage disorder, which was made in 8% of infants who underwent the procedure as a primary diagnostic investigation. Because of easy availability of BME, it is often utilized as a pre-screening technique in the evaluation of visceromegalic forms of sphingolipidoses like Gaucher's disease, Niemann–Pick disease, and related disorders. They are less specific, however, allows selected and economic use of less readily available but confirmatory biochemical and genetic investigations.Citation20
The diagnoses offered after BME was ‘megakaryocytic thrombocytopenia’ in 6.7% of infants who underwent this procedure as a primary diagnostic investigation. Four of these infants had isolated thrombocytopenia, while others had anemia and thrombocytopenia. BME was basically carried out to exclude malignancy and other BM failure syndromes, and to assess the megakaryocytic response. The presence of adequate number of megakaryocytes in these conditions is consistent with various causes resulting in destruction of platelets like immune thrombocytopenia, drug-induced thrombocytopenia, or infection-associated thrombocytopenia. During the same period, there were three patients with reduced megakaryopoiesis, one of whom presented with isolated thrombocytopenia and others with anemia and thrombocytopenia. The differential diagnosis of this includes congenital and acquired amegakaryocytic thrombocytopenia as well as BM failure syndrome.
The presence of increased histiocytes with hemophagocytosis in BM was reported in 13 children whose BME was performed for various indications including cytopenia. This is one of the eight criteria used in the diagnosis of HLH, however is not specific and can be seen in other conditions also, such as blood transfusion and sepsis. The presence of hemophagocytosis is a frequent finding in BM and there is no acceptable threshold above which it is considered significant. A recent study highlights these issues and has shown that the amount of hemophagocytosis in BMA or BMTB does not correlate with disease probability. However, its value cannot be questioned as it helps to exclude other diseases which may or may not be related to HLH.Citation13
BME helped to exclude specific pathologies including hematological malignancies, certain forms of storage disorder, PRCA, and other BM failure syndromes as well as various infiltrative disorders in nearly half of the BMEs performed as a part of primary diagnostic evaluation. BMTB is a difficult procedure in infants and our study shows that, in nearly 10% of BMEs the BMA was adequate for an opinion while the corresponding BMTB was not. In our experience, BMTB is an indispensable tool in the evaluation of infiltrative disorders like MSRCT, storage disorder, LCH, and NHL. The accurate cellularity of BM, the presence of fibrosis, and BM necrosis could be assessed only on BMTBs.
Conclusions
BME from PSIS comprising of aspirate as well as a trephine biopsy yields adequate samples in infants. Evaluation of cytopenias followed by suspicion of storage disorder remained the most common indications for BME procedure; and acute leukemias followed by storage disorder were the most common diagnoses offered in our study. BMTB is a difficult procedure in infants, however remains indispensable in situations where an infiltrative pathology is suspected. A specific pathology may not be seen in a large number of BMEs performed in this age group, however is an extremely valuable, quick, and economically viable procedure to confirm or exclude clinically suspected major hematological disorders in appropriate clinical settings.
Disclaimer statements
Contributors SS and MUSS designed the study, analyzed the data, and wrote the manuscript. NK, PS, SN, JA, RD and NV were involved in bone marrow examination and diagnosis. They reviewed the manuscript and made critical suggestions. RKM provided the patients for the study.
Funding None.
Conflicts of interest None.
Ethics approval The study has obtained approval from the institute ethical clearance committee.
References
- Bain BJ. Bone marrow trephine biopsy. J Clin Pathol. 2001;54:737–42.
- Bain BJ. Bone marrow aspiration. J Clin Pathol. 2001;54:657–63.
- Abla O, Friedman J, Doyle J. Performing bone marrow aspiration and biopsy in children: recommended guidelines. Paediatr Child Health 2008;13:499–501.
- Sola MC, Rimsza LM, Christensen RD. A bone marrow biopsy technique suitable for use in neonates. Br J Haematol. 1999;107:458–60.
- Bain BJ, Clark DM, Lampert IA, Wilkins BS. Bone marrow pathology. 3rd ed. Oxford: Blackwell Science Ltd; 2001.
- Lee SH, Erber WN, Porwit A, Tomonaga M, Peterson LC; International Council for Standardization in Hematology. ICSH guidelines for the standardization of bone marrow specimens and reports. Int J Lab Hematol. 2008;30:349–64.
- Harris NL, Campo E, Jaffe ES, Pileri SA, Stein H, Swerldlow SH, et al. Introduction to the WHO classification of tumours of haematopoietic and lymphoid tissues. In: Swerldlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. (eds.) WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. p. 18–30
- Reid MM, Roald B. Adequacy of bone marrow trephine biopsy specimens in children. J Clin Pathol. 1996;49:226–9.
- Means RT Jr, Glader B. Anemia: general considerations. In: Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Arber DA, et al. (eds.) Wintrobe's clinical hematology. 12th ed. Philadelphia, PA: Lippincot Williams & Wilkins; 2009. p. 780–809.
- Labarque V, Van Geet C. Clinical practice: immune thrombocytopenia in paediatrics. Eur J Pediatr. 2014;173:163–72.
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190–207.
- Beutler E, Saven A. Misuse of marrow examination in the diagnosis of Gaucher disease. Blood 1990;76:646–8.
- Ho C, Yao X, Tian L, Li FY, Podoltsev N, Xu ML. Marrow assessment for hemophagocytic lymphohistiocytosis demonstrates poor correlation with disease probability. Am J Clin Pathol. 2014;141:62–71.
- Ben-Baruch S, Canaani J, Braunstein R, Perry C, Ben-Ezra J, Polliack A, et al. Predictive parameters for a diagnostic bone marrow biopsy specimen in the work-up of fever of unknown origin. Mayo Clin Proc. 2012;87:136–42.
- Russell HV, Golding LA, Suell MN, Nuchtern JG, Strother DR. The role of bone marrow evaluation in the staging of patients with otherwise localized, low-risk neuroblastoma. Pediatr Blood Cancer 2005;45:916–9.
- Brown P. Treatment of infant leukemias: challenge and promise. Hematology Am Soc Hematol Educ Program 2013;2013:596–600.
- Orbach D, Sarnacki S, Brisse HJ, Gauthier-Villars M, Jarreau PH, Tsatsaris V, et al. Neonatal cancer. Lancet Oncol. 2013;14:e609–20.
- Baumann I, Bennett JM, Niemeyer CM, Thiele J, Shannon K. Juvenile myelomonocytic leukemia. In: Swerldlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. (eds.) WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. p. 82–4.
- de Vries AC, Zwaan CM, van den H-E. Molecular basis of juvenile myelomonocytic leukemia. Haematologica 2010;95:179–82.
- Ziyeh S, Harzer K. Bone marrow cytological storage phenomena in lipidoses. Eur J Pediatr. 1994;153:224–33.