Abstract
Objectives
This study focused on the expression pattern and clinical significance of B7-H3 expression in human acute leukemia.
Methods
We systematically analyzed 134 patients with acute myeloid leukemia (101 cases) and acute lymphocytic leukemia (33 cases) by flow cytometry.
Results
The frequency of B7-H3+ cases was 44.8% in total. The B7-H3 expression rate differed from 0% to 74.8% in individual cases. The correlation between B7-H3 expression and traditional prognostic factors, such as age and gender, the white blood cell count was not confirmed. However, B7-H3 had a significant higher expression in CD34+ cases and high risk karyotypes.
Conclusions
Owing to the expression of B7-H3 being statistically relevant in predicting disease progression and a shorter life survival, our results demonstrated that B7-H3 expression in acute leukemia predicts an unfavorable outcome.
Introduction
Acute leukemia (AL) represents a heterogeneous group of clonal bone marrow (BM) disorders arising as a result of progressive genetic damage occurring in hematopoietic progenitor cells.Citation1 Characterization of patients with AL according to presentation prognostic factors provides an important basis for selection of therapy. The common accepted factors infecting prognosis in AL are as follows: age, eastern cooperative oncology group (ECOG) performance status, white blood cell (WBC) counts, extramedullary infiltration, AL subtypes, molecular markers, cytogenetic characteristics, and response to treatments.Citation2,Citation3
Recently, more concerns are focused on immunophenotypic molecular expression on the malignant cells of leukemias.Citation4–Citation6 Aberrant antigen profiles of neoplastic population not only give us brand new insight into the pathogenesis of AL but are also of some diagnostic and prognostic value. Thus, specific antigen expression on malignant cells may play a role as another prognostic factor which gives therapeutic guidance.
T-cells play an important role in antitumor immunity. To ensure an appropriate T-cell response, two independent signaling pathways are finely tuned. The first signal requires recognition of the antigen-bearing major histocompatibility complex (MHC) on the surface of antigen-presenting cells (APCs) by the corresponding antigen-specific T-cell receptor (TCR) on T-cells. The second signal, which comes from independent antigen, is delivered by costimulatory molecules. The B7-1/B7-2:CD28/CTLA-4 signaling represents the best characterized costimulatory pathway.Citation7 Apart from stimulatory signals that augment and sustain T-cell responses, costimulatory pathways also deliver inhibitory signals that downregulate or terminate T-cell responses. Within the past two decades, new costimulatory ligands and receptors have been identified, including B7-H1 (programmed death-1 ligand-1), B7-DC (programmed death-1 ligand-2), PD-1 (programmed death-1), ICOS (inducible costimulator), ICOSL (ICOS-ligand), BTLA (B and T lymphocyte attenuator), B7-H3, and B7-H4.Citation8 These previously identified B7 homologues have been serving as novel modulators of antitumor immunity.
B7-H3 is a recently identified costimulatory molecule that has been implicated as a novel modulator of antitumor response.Citation9 B7-H3 expression related to clinical significance of various human cancers has been studied.Citation10–Citation14 However, its role in the regulation of T-cell response and in antitumor immunity remains controversial. Moreover, at present no study on hematological malignancies was reported. Therefore, our study focused on the expression of B7-H3 on blast cells of human AL and its relationship with clinical characteristics, aiming to find another potential molecular target for prognostic prediction and therapy.
Materials and methods
Patient populations
A group of 134 unselected patients with AL was studied for the expression of costimulatory molecule B7-H3. Of these, 73 male and 61 female patients with a mean age of 43 years (range, 1–85) were recruited. Informed consent was obtained from all participating subjects and the study was approved by the local ethics committee. BM samples were obtained at initial diagnosis before treatment from February 2011 until June 2012. The diagnosis of AL was established according to morphology, immunology, cytogenetics, and molecular biology. Acute myeloid leukemia (AML) patients were classified in accordance with the French–American–British (FAB) subtypes, that is, M0, 3; M1, 12; M2, 54; M3, 9; M4, 9; M5, 11; M6, 3. Acute lymphocytic leukemia (ALL) classification was in compliance with the World Health Organization (WHO)-2008Citation15 proposal, that is, 28 cases with B-lymphoblastic leukemia (B-ALL) and 5 cases with T-lymphoblastic leukemia (T-ALL). In all cases, diagnosis and classification of AL was made at least by two independent observers who were unaware of the B7-H3 expression results. BM samples from 12 healthy donors were grouped for control.
Risk stratification based on age, initial WBC counts, total blasts percentage in BM, and cytogenetic aberration was done at initial diagnosis. Correlation of these parameters and B7-H3 expression were studied in detail.
Immunophenotypic analysis
Anticoagulated BM samples were collected before therapy. Immunophenotypes were carried out on BM samples, which were directly analyzed at the time of collection. Mononucleated living cells were separated by centrifugation on a Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient and resuspended in phosphate-buffered saline (PBS). Subsequently, 1 × 106 cells were incubated at 4°C in 100 µl of cell suspension containing 20 µl specific antibody (as described below). After 20 minutes of incubation, cells were washed twice with PBS and for FACS analysis. Surface antigens were analyzed by three- or four-color immunofluorescence by combining phycoerythrin (PE)-, fluorescein isothyocyanate (FITC)-, and peridinium chlorophyll protein (PerCP)-conjugated monoclonal antibodies (mAbs). Sources and specificities of the mAbs used for immunophenotype and lineage assessment of AL blasts (recognizing the following antigens: CD33, CD13, CD117, CD34, CD15, CD7, CD3, CD4, CD8, CD2, CD5, CD10, CD19, CD22, CD20, CD14, CD11b, HLA-DR, CD56, CD61, CD62, CD64, CD41, CD42) have been reported in detail previously.Citation16 The PE-conjugated anti-B7-H3 mAb as well as PE-IgG murine isotype control mAb purchased from eBioscience (San Diego, CA, USA) was employed in combination with FITC-labeled anti-CD34 mAbs purchased from Pharmingen (BD Biosciences, USA). Viable, antibody-labeled blast cells were identified according to their forward and side scatter or CD45 expression and side scatter, electronically gated and analyzed on a FACSCalibur flow cytometer (BD Biosciences) by means of the CellQuest software (BD Biosciences, USA) collecting at least 10 000 events. A sample was classified B7-H3+ when at least 10% of the blast cells displayed a fluorescence intensity for B7-H3 compared with the same cell population stained with an isotype-matched control immunoglobulin.
Determination of B7-H3 mRNA expression
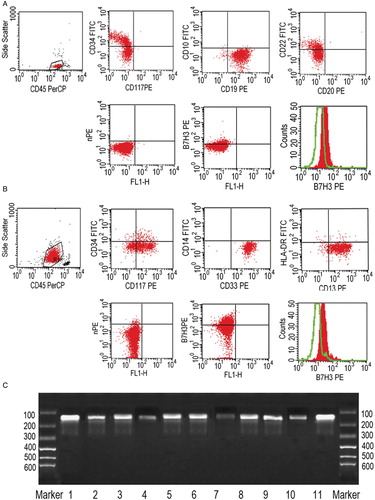
AL samples that contained ≥90% blast cells were selected for molecular studies. Total RNA was extracted using the guanidinium thiocyanate method (TRIzol; Invitrogen-Life Technologies, Paisley, UK). RNA (1 µg) was reverse transcribed into cDNA in a 20 µl reaction mix as described. cDNA (2 µl) was amplified by polymerase chain reaction (PCR) using the appropriate primers specific for B7-H3 (sense, 5′-ACA GAC CAC TGT GCA GCC TTA TTT C-3′; antisense, 5′-GGC AGC AGG CAG GAT GAC TTA-3′), using the following PCR conditions: 5 minutes denaturation at 94°C, 35 cycles of a denaturation step (94°C, 30 seconds), an annealing step (62°C, 35 seconds), an elongation step (72°C, 30 seconds), and a final extension of 10 minutes at 72°C. Six microliters of amplified PCR product (expected size 129 bp for human B7-H3) were separated by electrophoresis on 1.2% agarose gel and visualized by staining with ethidium bromide.
Statistical analysis
The association between the expression of B7-H3 and clinical data at diagnosis, i.e. age, gender, FAB or WHO classification, WBC, CD34 expression and cytogenetic risk classification, was studied by analysis of variance. Chi-square test was used for categorical variable data. Results were described as median ± standard deviation. For non-normal distributed values, the Mann-Whitney U test or Spearman rank test were performed for comparative statistical evaluations among groups and for correlation analysis with B7-H3 expression and clinical parameters. The Bonferroni correction was applied when multiple comparisons were performed. The univariate analysis and multivariate analysis of progression-free survival (PFS) and overall survival (OS) were performed using the Cox proportional hazards regression model. The stepwise and eliminate options using the likelihood ratio approach were used to assess the contribution of each of the variables in the regression model. PFS was calculated from the day when complete remission or partial remission was certified after a last chemotherapy until recurrence. OS, however, was referred to the date of diagnosis till the date of death or last follow-up (June 2013). Statistical analyses were done using the SPSS software package (SPSS 13.0). A P-value of 0.05 or less was considered statistically significant.
Results
Expression of B7-H3 molecule in AL patients and controls
B7-H3 surface antigen expression was detected on blast cells of AML and ALL cases. Selected samples were further assessed and certified at the mRNA level by PCR analysis of purified AL blasts. As shown in , the presence of an amplified product of the expected size (129 bp) was consistent with the flow cytometric data. A specific band could be detected in every subtype cases which were B7-H3+ in flow cytometry.
Figure 1. B7-H3 expression in representative AML and ALL cases. (A) Blast cells from an ALL case within an SSClow/CD45 expression electronic gate were gated out for analysis. Multicolor fluorescence analysis showed CD34+, CD19+, and CD22+ of these cells. Dot-plot represents a B7-H3 expression of cells stained with anti-B7-H3-PE mAb (y-axis) compared with those stained with PE-IgG murine isotype control mAb (y-axis). A detectable level of B7-H3 was revealed by comparing the staining cells with anti-B7-H3-PE mAb (shaded histogram) with PE-IgG murine isotype control mAb (open histogram). (B) Blast cells from an AML case showed CD117+, CD13+, and CD33+. Dot-plot and histograms both represent a B7-H3 expression on blast cells (as previous description). (C) Purified AL blasts from AL subtype cases were analyzed at the mRNA level by PCR. Bands with 129 bp size exhibited different densities at lane 1–11 (B-ALL, M1, M2, M3, M4, M5, T-ALL, M2, B-ALL, M5, B-ALL), which were the expected amplified product of the B7-H3 molecule.
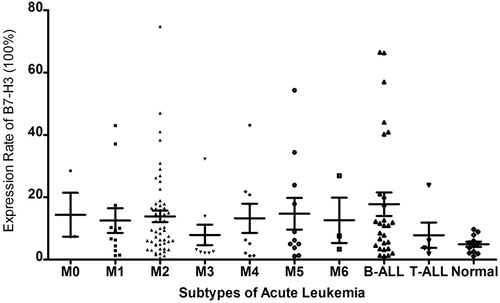
By establishing a cut-off level at 10% of blasts coexpressing B7-H3, the overall frequency of B7-H3+ cases was 44.8% (60/134). We did not observe apparently differences of B7-H3 expression in normal samples compared with the murine IgG mAb isotype control (1.1–9.7%). B7-H3 positive expression was heterogeneous within individuals with a range of 10.0–74.8%. The positive rate and expression range of B7-H3 in AL subtypes were as follows: M1: 42% (1.3–43.0%), M2: 50% (0.5–74.8%), M3: 22% (2.1–32.3%), M4: 44% (1.2–43.1%), M5: 36% (1.1–54.3%), B-ALL: 54% (1.1–66.5%), and T-ALL: 20% (1.9–23.9%). Though a relative lower expression rate and distribution of B7-H3+ cases within M3 (2/9) and T-ALL (1/5) subtypes are observed in , there were no significant differences observed compared with other FAB subtypes, neither of expression rate nor of positive case distribution. Statistical analysis proved to be of no support of a preferential high expression in M2 (27/54) or B-ALL (15/28), either. Confined in our study, we did not observe a special expression of B7-H3 in any subtype.
Relationship between B7-H3 expression and various prognostic factors
Among the 134 AL patients, all FAB subtypes except for M7 were represented. showed the relationship between clinical characteristics and B7-H3 expression. Among the 134 cases, no significantly higher frequency of B7-H3+ cases was found in the male or female group (P = 0.913). Neither was observed between patients who were older than 60 years and those less than 60 years (P = 0.383). Furthermore, no correlation could be obtained between B7-H3 expression and percentage of blast cells in BM (P = 0.756). WBC counts were recorded in 112 cases and in total, the B7-H3 positive expression was of no significance within the higher WBC subset (a cut-off level of 30 × 109/L, P = 0.252).
Table 1. The chi-square test of clinical characteristics and B7-H3 expression
Conventional banded cytogenetic studies of pretreatment BM were available for 108 (80.6%) of the 134 patients, with 83 AML and 25 ALL, respectively. Distribution of the cytogenetic risk status according to the Southwest Oncology Group (SWOG) classifications certificated the unfavorable karyotypes distributed significantly in B7-H3+ groups (P = 0.034). Separately, in ALL group, 10 unfavorable cases with t(9;22)/Ph+ and complex karyotypes were of no significant B7-H3 positive expression cases (P = 0.187) than 15 intermediate ones who had normal karyotypes. However in the AML group, though there were no significant differences when comparing B7-H3 positive cases in 10 favorable cases with 56 intermediate ones (P = 0.724) and 17 unfavorable ones (P = 0.078), statistical finding was confirmed in the latter two (P = 0.035).
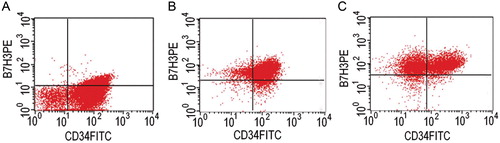
B7-H3+ cases coexpressing with a profile of surface antigens were as follows: CD34(42/60), CD117(34/60), HLA-DR(47/60), CD13(46/60) and CD33(48/60), CD7(12/60), CD10(8/60), CD11b(14/60), CD14(1/60), and CD15(15/60). As shown in , the patterns of B7-H3 positive expression as well as CD34 on blast cells of AL were generally classified into three types. Most of the B7-H3-positive cases exhibited the features of A. CD34+ cells preferentially expressed the B7-H3 molecule. High expressions of B7-H3 or expressing on CD34− cells, like B and C, were individual, accounting for less than 10% of the total cases. A significant correlation between B7-H3 and CD34 expression was confirmed (P = 0.000). Patients belonging to the CD34+ group had more B7-H3+ cases (P = 0.032) than those belonging to the CD34− group.
Figure 3. B7-H3 and CD34 coexpression patterns. Cells from AL cases were double-stained with anti-B7-H3-PE (y-axis) and anti-CD34-FITC (x-axis) mAbs and analysed by flow cytometry. Each dot-plot shows a representative pattern for B7-H3/CD34 expression. B7-H3 almost exclusively expressed on CD34-positive cells and most AL cases were of a weak positive expression as shown in (A). High expressions of B7-H3 (B) and the expression on CD34− cells (C) were individual.
B7-H3 expression predicts a short survival in AL
Follow-up on PFS was available in 80 of 134 AL patients. The median ± standard deviation of PFS in 40 B7-H3 positive cases was 5.0 ± 5.272 (range 0–23) months, compared with 6.0 ± 7.547 (range 0–24) months in 38 B7-H3 negative cases. Likewise, patients belonging to the B7-H3+ group had shorter median ± standard deviation of OS (8.25 ± 5.435, range 0.5–22 months) than patients belonging to the B7-H3− group (9.0 ± 7.730, range 0.3–26 months). As shown in Tables and , univariate analysis demonstrates that B7-H3 expression, subtype, and cytogenetics identified a cohort of patients that are more prone to disease progression (P = 0.01, P = 0.011, P = 0.012 separately). B7-H3 expression and cytogenetics also suggested a cohort of patients with shorter survival (P = 0.003 and P = 0.011). However, our multivariate analysis found that only B7-H3 expression as an independent factor when it was analyzed in independent data sets involving AL subtype, cytogenetic risk status, and B7-H3 expression.
Table 2. Univariate and multivariate Cox regression analyses of clinical features, B7-H3 expression in relation to progression-free survival
Table 3. Univariate and multivariate Cox regression analyses of clinical features, B7-H3 expression in relation to overall survival
Discussion
Within the last decade, there has been an upsurge of new insights into fundamental regulatory mechanisms governing host immune cell activation and function. As a result, a number of potent and relatively novel immunotherapeutic manipulations have recently emerged that hold great promise for the treatment of human malignancy.Citation17 In general, the primary goal of antitumoral immunotherapy is to generate a systemic antigen-specific T-cell response that is capable of destroying a tumor, its metastases, or even the parent tissues from which tumors arise. T lymphocytes are able to recognize and specifically respond to an incredible variety of foreign and native antigens. To ensure an appropriate T-cell response, which is essential to eradicate pathogens and to maintain self-tolerance, T-cell activation is finely tuned by two independent signaling pathways.Citation18 The first signal requires recognition of the antigen-bearing MHC on the surface of APCs by the corresponding antigen-specific TCR on T-cells. The second signal, which is antigen independent, is delivered by costimulatory molecules.Citation18 The B7/CD28 family represents the best characterized costimulatory pathway.Citation19 Recently, some previously identified B7 homologues have been implicated as potential regulators of antitumor immunity.Citation20–Citation22 For example, aberrant B7-H1 expression by cancer cells has been associated with adverse pathologic features and poor outcome in different human malignancies and has therefore been postulated as a potential mechanism by which malignant tumors may evade host immune response.Citation23,Citation24
B7-H3 is a type I transmembrane protein that shares 20–27% amino acid identity with other B7 family members.Citation9 It is another recently identified costimulatory molecule that has been implicated as a potential regulator of the antitumor response. B7-H3 is not only broadly expressed in peripheral healthy tissues, such as osteoblasts, fibroblasts, fibroblast-like synoviocytes, and epithelial cells, as well as in human liver, lung, bladder, testis, prostate, breast, placenta, and lymphoid organs,Citation17 but also found in a variety of different human solid tumors, including prostate cancer, clear cell renal cell carcinoma (CCRCC), non-small-cell lung cancer (NSCLC), pancreatic cancer, gastric cancer, ovarian cancer, colorectal cancer, and urothelial cell carcinoma.Citation10–Citation13,Citation25–Citation28 Since both stimulatory and inhibitory properties have been identified and both beneficial as well as adverse effects of B7-H3 expression in cancers have been reported, its role in the regulation of T-cell response and in antitumor immunity remains ambiguous.
Loos et al.Citation11 reported 88.2% of pancreatic cancer cells in a series of 68 cases that expressed B7-H3 molecule, and high tumor B7-H3 expression was associated with significantly better postoperative prognosis. Support of the possible stimulatory function of B7-H3 in antitumor responses also comes from a retrospective analysis in gastric cancer performed by Wu et al.,Citation12 where 58.8% of gastric cancer cells in a series of 102 patients have been shown to express B7-H3 in the cell membrane and cytoplasm and its expression positively correlated with survival time, infiltration depth, and tissue type. However, several studies in other human cancers correlating tumor B7-H3 expression with clinicopathological features do not concur with these findings. With 37.1% expression rate of tumor cells, B7-H3 in 70 patients with NSCLC inversely correlated with the number of tumor-infiltrating lymphocytes (TILs) and significantly correlated with lymph nodemetastasis.Citation10 Afterwards, another two studies that investigated B7-H3 expression in 823 and 338 patients with prostate cancer, respectively, showed some results. Strong B7-H3 expression in the resected specimens correlated with disease spread, poor clinicopathologic features, as well as cancer progression and recurrence.Citation28,Citation29 In CCRCC, 17.4% of tumor cells and 95.1% of tumor vasculature in 743 examined patients expressed B7-H3. B7-H3 expression in either tumor cells or tumor vasculature was found to significantly associate with an increased risk of death from CCRCC.Citation25 Most recently, B7-H3 was found to be expressed in 93% of 103 examined ovarian borderline tumors and carcinomas. B7-H3 was also expressed in the endothelium of tumor-associated vasculature in 44% of patients. Carcinomas with B7-H3-positive tumor vasculature were associated with a significantly shorter survival time and a higher incidence of recurrence.Citation13 To our knowledge, little information is available on the distribution and clinical significance of B7-H3 in human hematopoietic malignancies, with a special focus on acute leukemias. Therefore we sought to examine the expression of B7-H3 in AL and its association with clinical outcome.
As earlier, B7-H3 is reported to be broadly expressed in peripheral healthy tissues. However, in BMs obtained from those healthy volunteer donors, flow cytometry could not display positive expression of B7-H3 whether in lymphocytes or in granulocytes. There were no bands in PCR examination either. In regard to a positive expression of surface antigen when describing hematological malignances, a standard cut-off of 20% was accepted by all. However, some reported studies also identified a sample as positive when as few as 5–10% of cells were stained over their respective controls.Citation30,Citation31 Likewise, a cut-off of 10% of positive cells was employed to distinguish between B7-H3+ and B7-H3− cases in our research. With this regard, 44.8% (60/134) cases were found positive. Distribution of B7-H3+ cases in AL subtypes was of no statistical differences in our study. Since there is a relatively lower expression rate and distribution cases in M3 and T-ALL groups, we do not exclude the possibility, due to our small scale samples, that there is an expression specialty of B7-H3 in other AL subtypes.
Most of our AL cases exhibited their B7-H3 antigen in a dimly positive way though the B7-H3 molecule was more prone to be expressed on progenitor or blast cells. According to the results by flow cytometry, we found that most B7-H3 was expressed in the CD34+ cell fraction. In a more complete flow cytometric profile, the 60 cases of B7-H3+ AL were coexpressed with CD34, the other progenitor markers CD117 and HLA-DR, as well as the myeloid antigens CD13 and CD33 in most cases. On the contrary, lymphoid antigens (CD7, CD10) or antigens representing a more mature stage (CD11b, CD14, and CD15) were either mainly negative or variably expressed by B7-H3+ AL blasts. Therefore, conclusion arising from above result is that B7-H3 may be one of the markers expressed during the early stage, which will vanish on maturation.
By analyzing the association of B7-H3 expression and the characteristics of AL cases of our series, some observations emerged. First, we demonstrated that the B7-H3 expression was of no obvious relevance with the traditional prognostic parameters like gender, age, and WBC counts. Second, the expression rate of B7-H3 was significantly higher in the group of CD34+ patients. The effect of surface markers of CD34 on prognosis was variable. Some studies reported it has had no impact on the outcome,Citation32 while some reported it is expressed in favorable AML subtypes, including AML with t(8;21) and inv(16).Citation33 Conversely, the CD34 expression is also found to be associated with prognostically adverse karyotypes, including abnormalities of chromosome 5 (−5/5q−) and 7(−7/7q−).Citation34 We did not examine the CD34 expression on the prognosis of our study in detail. However, our findings were in accordance with previous reports on the negative function of B7-H3, which predicts poor outcomes. This corroborates the notion that these specific B7-H3+ AL subsets might share several clinical and biological features, including a worse prognosis and a phenotype of blast cells more often expressing CD34. Third, the correlation between B7-H3 expression and cytogenetics was another finding of our study. Among the 108 AL patients with informative cytogenetics, B7-H3+ cases fell within the group of patients carrying high-risk karyotypes.
Though our follow-up did not overlap the whole patients and underwent a relatively short period, statistical data gave the impression that the group of B7-H3-positive patients had a high risk of disease progression or recurrence, as well as a short life survival. The B7-H3+ group has a mean survival of 9 months and PFS of 6 months. In contrast, the B7-H3− group has a mean survival of 16 months and PFS of 15 months, which was almost two-fold longer.
In this paper, for the first time, we demonstrated the expression pattern of B7-H3 in hematological malignancies, especially in the AL group. B7-H3 was a dimly positive surface marker on blast cells of AL, which appeared in the premature stage. Of no different distribution in AL subtypes, B7-H3 expression significantly correlated with adverse prognostic parameters and predicts a poor outcome. Although further and more studies would certainly be required to better understand the exact role of B7-H3 in AL, as well as in other hematological malignancies, our data suggested that treatment to the target of B7-H3 might represent a promising approach to improve the outcome of AL patients.
Acknowledgement
We express appreciation for the assistance given by the clinicians, nurses, and laboratory workers at these hospitals. We are indebted to Wujun Jiang for his assistance in the statistical analysis, and to the patients and their families.
Disclaimer statements
ContributorsHQ was responsible for the study design; obtaining funding; logistic, administrative, and technical support and data interpretation; drafting, critical revision, and final approval of the article. YW, JX, LW, and WC were responsible for the recruitment of participants and data collection. YH and XL were responsible for data analysis and paper writing. WZ, JL, and SZ were responsible for drafting, critical revision, and for the final approval of the article. HQ is the guarantor.
Funding The study was supported by the National Natural Science Foundation of China (Funds Number: 81070456, 81270652).
Conflicts of interest None.
Ethics approval The study was approved by the Ethics Committee of Nanjing Medical University.
References
- Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Hematol Oncol Clin North Am. 2010;24:35–63.
- Estey EH. Acute myeloid leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87:89–99.
- Grimwade D, Hills RK. Independent prognostic factors for AML outcome. Hematol Am Soc Hematol Educ Program 2009;1:385–95.
- Dalal BI, Mansoor S, Manna M, Pi S, Sauro GD, Hogge DE. Detection of CD34, TdT, CD56, CD2, CD4, and CD14 by flow cytometry is associated with NPM1 and FLT3 mutation status in cytogenetically normal acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2012;12:274–9.
- Iwamoto S, Deguchi T, Ohta H, Kiyokawa N, Tsurusawa M, Yamada T, et al. Flow cytometric analysis of de novo acute lymphoblastic leukemia in childhood: report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol. 2011;94:185–92.
- Cheuk AT, Wells JW, Chan L, Westwood NB, Berger SA, Yagita H, et al. Anti-tumor immunity in a model of acute myeloid leukemia. Leuk Lymphoma 2009;50:447–54.
- Abbas AK. The control of T cell activation vs. tolerance. Autoimmun Rev. 2003;2:115–8.
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48.
- Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2:269–74.
- Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer 2006;53:143–51.
- Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer 2009;9:463.
- Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, et al. Relationship between costimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12:457–9.
- Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23:1104–12.
- Boorjian SA, Sheinin Y, Crispen PL, Lohse CM, Leibovich BC, Kwon ED. T-cell co-regulatory molecule expression in renal angiomyolipoma and pulmonary lymphangioleiomyomatosis. Urology 2009;74:1359–64.
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the WHO classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009;114:937–51.
- Gattei V, Degan M, Gloghini A, De Iuliis A, Improta S, Rossi FM, et al. CD30 ligand is frequently expressed in human hematopoietic malignancies of myeloid and lymphoid origin. Blood 1997;89:2048–59.
- Loos M, Hedderich DM, Friess H, Kleeff J. B7-H3 and its role in antitumor immunity. Clin Dev Immunol. 2010;2010:1–7.
- Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res. 2008;103:1220–31.
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–26.
- Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6:223.
- Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA 2003;100:10388–92.
- Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, et al. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med. 2003;197:1721–30.
- Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109.
- Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006;8:190–8.
- Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–7.
- Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, et al. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163–71.
- Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res. 2008;14:4800–8.
- Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–900.
- Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci. 2007;104:19458–63.
- Holden JT, Geller RB, Farhi DC, Holland HK, Stempora LL, Phillips CN, et al. Characterization of Thy-1(CDw90) expression in CD34+ acute leukemia. Blood 1995;86:60–5.
- Kozii R, Wilson J, Persichetti J, Phelps V, Ball SE, Ball ED. Thy-1 expression on blast cells from adult patients with acute myeloid leukemia. Leuk Res. 1997;21:381–5.
- Kyoda K, Nakamura S, Hattori N, Takeshima M, Nakamura K, Kaya H, et al. Lack of prognostic significance of CD34 expression in adult AML when FAB M0 and M3 are excluded. Am J Hematol. 1998;57:265–6.
- Fruchart C, Lenormand B, Bastard C, Boulet D, Lesesve JF, Callat MP, et al. Correlation between CD34 expression and chromosomal abnormalities but not clinical outcome in acute myeloid leukemia. Am J Hematol. 1996;53:175–80.
- Fagioli F, Cuneo A, Carli MG, Bardi A, Piva N, Previati R, et al. Chromosome aberrations in CD34-positive acute myeloid leukemia: correlation with clinicopathologic features. Cancer Genet Cytogenet. 1993;71:119–24.