Abstract
Objectives
Derived from plants, flavonoids have been proven to possess anti-cancer activities. Adriamycin (ADM), an anthracycline antibiotic, is widely applied in the chemotherapy for leukemia; however, it has a side effect of heart damage. This study aims to explore potential anti-leukemia effects of quercetin (Que) and the underlying mechanism.
Methods
The P388 xenograft mice models were first established and then treated with Que alone or in combination with ADM. Subsequently, we evaluated their effects on cell proliferation and apoptosis by observing the cell cycle and detecting the Caspase-3 level, respectively. The underlying pro-apoptotic mechanism was further investigated by detecting the expression levels of NF-κB, Bcl-2, and Bax. The cardiomyocyte ultrastructural changes of P388 leukemic mice after drug treatment were also observed. The protective effect of Que on cardiomyocyte was evaluated by detecting enzymatic activity changes of glutathione peroxidase, superoxide dismutase, and malondialdehyde.
Results
Compared with ADM group, the combination of ADM and Que showed prolonged survival time and less peripheral white blood cells. Que could sensitize the anti-leukemic effect of ADM by inhibiting the proliferation of white blood cells through trapping the cells at the S phase; caspase-3 was activated via the expressional regulation of Bcl-2, Bax, and NF-κB. When applied in combination with ADM, Que could attenuate heart damage by cleaning the reactive oxygen species.
Conclusion
Our study may provide informative evidences for the underlying mechanism of anti-cancer effects of Que and sheds light on the clinical application of Que in leukemia treatment.
Introduction
Leukemia is a blood or bone marrow cancer type with abnormal increase of immature white blood cells.Citation1 Clinically and pathologically, leukemia is subdivided into acute lymphoblastic leukemia, chronic lymphocytic leukemia, acute myelogenous leukemia, and chronic myelogenous leukemia. Leukemia patients often display impaired blood clotting process, weak immune system, and neurological symptoms.Citation2 Every year ∼256 000 people around the world develop some form of leukemia, and 209 000 people die from it.Citation3 About 90% of the leukemias are diagnosed in adults. Most forms of leukemia can be treated by chemotherapy, medical radiation therapy, hormone treatments, or bone marrow transplant.Citation4,Citation5 Adriamycin (ADM), an anthracycline antibiotic, is widely applied in chemotherapy of many cancer types, including leukemia.Citation6 ADM works by intercalating DNA.Citation7,Citation8 However, ADM had the most dangerous side effect of heart damage,Citation9–Citation11 which is due to the myocyte damage caused by reactive oxygen species (ROS) generated from the interaction between ADM and iron.
Fruits and vegetables contain many bioactive compounds, which have been proven to be responsible for reducing the risk of common human cancers. Increasing interest has been paid to flavonoids, a large group of compounds with a similar structure, which consists of two phenolic benzene rings linked to a heterocyclic pyre or pyrone.Citation12 Recently, quercetin (Que), (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one), a kind of flavonoid, has been reported to possess anti-cancer effects.Citation13 It has been reported that the uptake of Que has an inverse association with the incidence of pancreatic cancer and lung cancer in current smokers.Citation14 Although there is no direct evidence for the interaction of Que with cellular receptors, various cellular receptors have been proven to be involved in the cancer-preventive property of Que, such as the 67 kDa cell surface laminin receptor,Citation15 insulin-like growth factor-I,Citation16 vimentin,Citation17 and an aryl hydrocarbon receptor.Citation18,Citation19 Que also modifies signal transduction during carcinogenesis, including cell cycle regulation, apoptosis, pro-inflammatory protein induction, and angiogenesis.
However, there is no relative study about potential effects of Que on leukemia treatment. Here, we investigated the anti-leukemia effects of Que and its potential sensitization effects to ADM in a P388 xenograft nude mice model. We also investigated the underlying mechanism by determining the levels of Caspase-3, Bcl-2, Bax, NF-κB, and the activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and malondialdehyde (MDA). Our study may provide informative evidences for the mechanism exploration of the anti-cancer effects of Que and sheds light on the clinical application of Que in leukemia treatment.
Materials and methods
All animal studies have been approved by The Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University and performed in accordance with the ethical standards.
Cell culture
P388 leukemia cells (Ruiqi Biotechnology Company, Shanghai, China) were cultured in RPMI1640 (Hyclone, Shanghai Bioleaf Biotech Co., Ltd, Shanghai, China) medium containing 10% fetal bovine serum (Hyclone) at 37°C in 5% CO2 (incubator: Thermo Electron Corporation, Cambridge, UK; ultra-clean bench: Beijing Guanpeng Purification Equipment Co., Ltd, Beijing, China). The cells were collected at the exponential phase.
Leukemia mice model design
Seventy SPF Balb/c nude mice (35 males and 35 females) were purchased from Beijing Vital River Laboratories Company (certificate No.: SCXK 2006-0009) and raised in SPF biological laminar flow rack at the Clinical Medical Research Center of the Affiliated Hospital of Inner Mongolia Medical University.
The P388 cells at the exponential phase were collected and washed with phosphate-buffered saline (PBS; pH 7.4) twice, then re-suspended (refrigerated centrifuge: Sigma, Osterode, Germany) with PBS at 106 cell/ml, and finally subcutaneously injected into the left anterior axilla of the mice (0.2 ml for each).
Experimental grouping and medication method
Que (Sigma, St. Louis., MO, USA) was dissolved in dimethyl sulfoxide (DMSO, Beijing Chemical Reagent Company, Beijing, China). ADM (Shenzhen Main Luck Pharmaceuticals, Inc., Shenzhen, China) was prepared in physiological saline (PS). After the elimination of the mice with no xenograft or abnormal tumor size, the remaining 60 mice with tumor diameter of 0.8 cm were randomly divided into six groups: control, low Que, high Que, ADM, ADM with low Que, and ADM with high Que for peritoneal injection lasting for 15 days ().
Table 1. Medication methods for six P388 leukemic nude mice groups
Tail blood was collected before and after P388 inoculation at Weeks 0, 2, 3, and 4 from 2 days after medication for peripheral blood hemogram and white blood cell number determination.
Life prolongation rate
The survival time was counted from the day when mice were inoculated. Life prolongation rate and sensitization were calculated according to the following formulae: life prolongation rate = (average survival time of experimental group/average survival time of control group − 1) × 100%; sensitization = average survival time of experimental group/average survival time of control group.
Cell cycle detection assay
Tumor tissues of the mice were minced and centrifuged at the end of Week 4, then, the cell suspension was transferred into a BD flow cytometry tube. The cells were washed with 500 µl 1× PBS and digested with 500 µl 0.25% trypsin. The liquid in the tube was then collected and centrifuged at 2000 rpm, 4°C for 8 minutes. Next, the cells were re-suspended in 1 ml 1× PBS. While gently vortexing the suspension, 100 µl 1× PBS and 900 µl 90% ethanol were added in a drop-wise manner. After fixing at 4°C for 2 hours, the cells were collected after centrifugation and washed with 1 ml 1× PBS. Finally, 300 µl PI dye was used for staining at room temperature for 10–15 minutes. Cell cycle analysis was performed with Flow Cytometry (Beckman Coulter, Inc., Fullerton, CA, USA).
Apoptosis analysis
Tissues were minced, weighted, and homogenized manually in proper volume of, pH 7.4, PBS at the end of Week 4. After centrifugation at 2000–3000 rpm for 20 minutes, the supernatant was carefully collected and used for apoptosis analysis according to the manual of Annexin V-FITC ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The sample concentration was determined by measuring the optical density (OD) value at 450 nm with a microplate reader (Thermo Electron Corporation, Cambridge, UK).
Real-time polymerase chain reaction
The total RNA was extracted according to the manual of Trizol (Tiangen Biotech, Co., Ltd, Beijing, China). The RNA concentration and purity was determined with a trace nucleic acid analyzer. The total RNA (2 µg) was used for reverse transcription (Tiangen Biotech, Co., Ltd) in accordance to the following condition: 20 µl system, 70°C for 5minutes, 2 minutes on ice, 25°C for 10 minutes, 42°C for 50 minutes, 95°C for 5 minutes. The cDNA template was used for real-time PCR (Real-Time PCR kit: Tiangen Biotech, Co., Ltd, ABI7300 Real-Time PCR Instrument: ABI) using the condition below: 10 µl system, 95°C for 10 seconds, 55°C for 20 seconds, 72°C for 31 seconds, 40 cycles. Primers used are listed in . Using GAPDH as an internal reference, relative contents for Bcl-2, Bax, and NF-κB were calculated using the 2−△△Ct method: △Ct = Cttest gene = CtGAPDH; △△Ct = △Ct (Experimental group) = △Ct (DMSO control group); fold change N = 2−△△Ct.
Table 2. Real-time PCR primers for Bax, Bcl-2, NF-κB, and GAPDH
Western blotting
The cells were washed twice with cold PBS and applied with 50 µl lysis buffer on ice for 20 minutes. The cells were then collected, and transferred into a pro-cold 1.5 ml EP tube on ice for 1 hour. Subsequently, the total protein supernatant was obtained by centrifugation (10 000 rpm at 4°C for 10 minutes) and used for concentration determination via the Bradford method. Loading samples were prepared by adding an equal volume of 2× sodium dodecyl sulfate buffer and incubating in boiling water for 5 minutes, and then sodium dodecyl sulfate–polyactylamide gel electrophoresis separation was performed at 120 V for 15 hours. Protein bands were transferred onto nitrocellulose membrane (NCM) with electroporator at 58 V, 4°C for 4 hours. The NCM was subsequently incubated at room temperature in 1:200 primary antibodies and 1:500 secondary antibodies for 2 and 1 hour, respectively. All the antibodies (anti-Bcl-2, Bax, NF-κB p65, and β-Ainten) were purchased from Santa Cruz. NCM was developed by adding diaminobenzidine (DAB) substrate for 20–30 minutes. The reaction was terminated by washing the NCM with water. Immunoblotting for the internal reference β-tubulin was conducted simultaneously. The digital images were taken after DAB visualization. The images were analyzed with the GDS8000 Image Analysis System. The average OD value of a certain protein band was used to indicate the protein expression level.
Ultrastructure changes of cardiomyocyte
Two weeks after medication, the myocardial tissue from the left ventricle was minced into <1 mm explants. The samples were fixed in 3% glutaraldehyde and 1% osmic acid, sequentially. After dehydration, fixed explants were embedded with Epon812 and sliced, followed by uranium lead double staining, the myocyte ultrastructural changes were observed under electron microscopy.
GSH-Px activity detection
The 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) colorimetric technique was utilized. One gram fresh myocardium was homogenized in 25 ml of 5 mM EDTA-TCA solution, and then 2 ml of solution was extracted and mixed with 0.4 ml of 1 mM NaOH solution, 1.5 ml of 0.2 M pH 7.0 potassium phosphate buffers, and 0.1 ml of DTNB reagent. The mixture was developed at room temperature for 5 minutes. Distilled water was added to the mixture to 5 ml in total and OD at 412 mm was measured. The blank control was set by replacing DTNB with potassium phosphate buffer. Reduced form of GSH was used as a reference standard.
SOD and MDA activity detection
Mice hearts were first minced and then homogenized with nine volumes of PS. After centrifugation at 3000 rpm for 5 minutes, the supernatant was used to detect SOD content and MDA content with the hydroxylamine method and the thiobarbituric acid method in accordance with the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively.
Data analysis
SPSS13.0 was used for statistical analysis. Data of all the groups were expressed as average value ± standard deviation. Paired comparisons among groups were performed with one-way analysis of variance. P < 0.05 was considered to indicate statistical significance.
Results
Que prolongs the survival time of P388 xenograft Balb/c nude mice
After being inoculated with P388 cells for 2 weeks, obvious bulges were seen underneath the skin of the left anterior axilla (A), and 4 weeks later, the mice displayed different degrees of mental fatigue, accidie, wrinkled fur, plump abdomen, and appearances of xenograft tumor (B). Compared with DMSO control group, low Que, high Que, ADM, ADM with low Que, and ADM with high-Que group showed lifetime prolongation rates and sensitizations of 58.96% and 0.62, 79.10% and 0.56, 57.46% and 0.65, 83.58% and 0.55, and 105.22% and 0.51, respectively ().
Figure 1. P388 xenograft mice models. (A) Nude mice images taken 2 weeks after P388 cell inoculation; (B) nude mice images taken 4 weeks (B) after P388 cell inoculation.
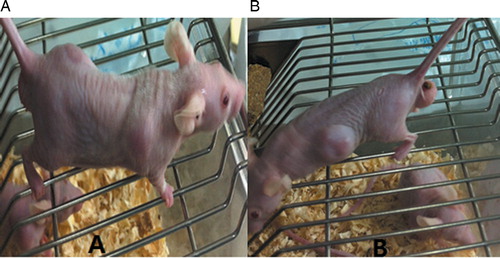
Table 3. Survival time summary for control and experimental groups
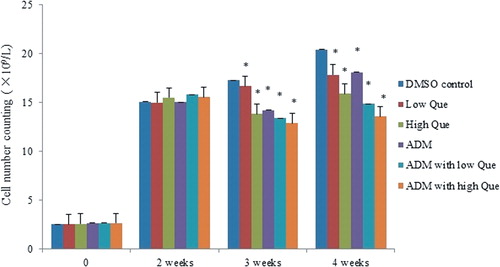
Que could reduce white blood cells of peripheral blood in P388 xenograft Balb/c nude mice
The number of white blood cells in the peripheral blood of P388 leukemic mice had been increasing significantly 2 weeks later since P388 inoculation. After Que and ADM medication, the white blood cell number of each medication group decreased, of which the ADM with high-Que group showed the most significant change ().
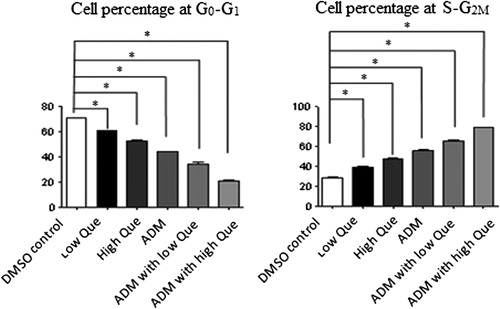
Que changes cell cycle of P388 xenograft Balb/c nude mice
The Flow Cytometry data showed the portion of cells at the G0–G1 phase at each of the medication group decreased significantly (P < 0.05), while cells portion at the S phase and the G2–M phase remarkably increased (P < 0.05) when compared with DMSO control group (). ADM with low-/high-Que groups displayed the most significant changes.
Que affects apoptosis by regulating Caspase-3 activity
Compared with DMSO control group, low-Que group showed enhanced Caspase-3 level, while high-Que group displayed reduced Caspase-3 level (A). High-Que group had similar low Caspase-3 level as ADM group. There is no significant Caspase-3 level difference between high-Que and low-Que groups when they were applied in combination with ADM.
Figure 4. Que activates Caspase-3 by regulating the expression of BAX, Bacl-2, and NF-κB. (A) Caspase-3 activity comparisons among control group and medication groups. Data were generated from Caspase-3 ELISA; (B) BAX mRNA level in different groups; (C) Bcl-2 mRNA level in different groups; (D) NF-κB mRNA level mRNA level in different groups; (E) BAX, Bacl-2, and NF-κB protein level comparisons among control and medication groups. aComparison with DMSO control group P < 0.05; bcomparison with low-Que group P < 0.05; ccomparison with ADM group, P < 0.05; dcomparison to ADM with low-Que group, P < 0.05.
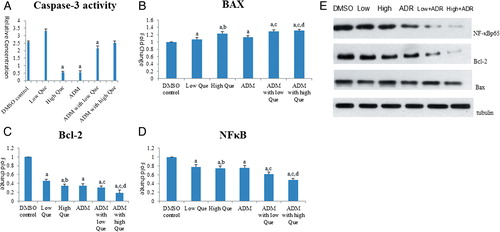
Que could affect apoptosis by regulating the expression of Bcl-2, Bax, and NF-κB p65
Real-time polymerase chain reaction (PCR) results showed that compared with DMSO control group, all the medication group showed enhanced Bax mRNA level (B) and decreased Bcl-2 (C) and NF-κB p65 mRNA (D) levels. The western blot results (E) showed that Bax protein levels of low Que and ADM with high-Que group were slightly increased compared with DMSO control group (P < 0.05), and protein levels of Bcl-2 and NF-κB p65 from the medication groups were reduced significantly in a dose-dependent manner, of which ADM with Que group showed the most significant changes (P < 0.05).
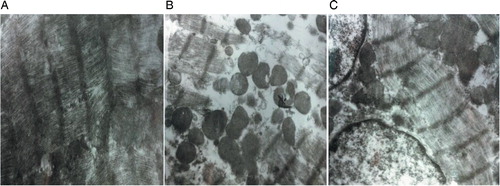
Que could alleviate the side effect of ADM by protecting cardiomyocyte
Transmission electron microscopy (TEM) images showed that the ADM group had series of cardiomyocyte damages, such as uneven myocardial fibers arrangement, fuzzy boundaries, myocardial fiber disorders, break-outs, disintegrations, and increased mitochondrial vacuolars (B) compared with DMSO control group (A). When ADM was applied with Que, the damages to cardiomyocytes were alleviated to some degree (C).
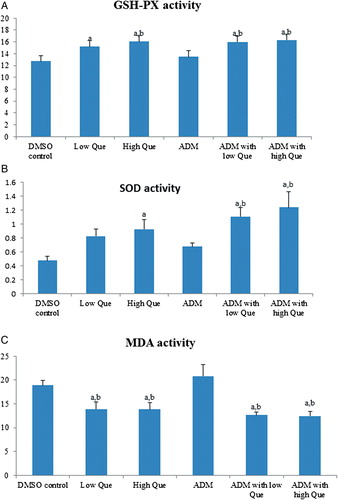
Que regulates activities of GSH-Px, SOD, and MDA
There was no difference between DMSO control and ADM group in terms of GSH-Px (A), SOD (B), and MDA (C) activities. Except ADM group, all the Que groups had higher GSH-Px and SOD activities, and lower MDA activity when compared with either DMSO control or ADM group.
Figure 6. Que removes ROS by regulating GSH-Px, SOD, and MDA activities. (A) GSH-Px enzymatic activity comparisons among all groups; (B) SOX enzymatic activity comparisons among all groups; (C) MDA enzymatic activity comparisons among all groups. aComparison with DMSO control group, P < 0.05; bcomparison with ADM group, P < 0.05.
Discussion
With generally less hazards to safety than synthetic compounds, there is a growing trend and increasing market for natural bioactive compounds extracted from fruits and vegetables. Botanical products are being used as functional foods, food supplements, flavors, and cosmetics. There are laboratory studies investigating Que's potential for anti-cancer applications.Citation12,Citation13,Citation20–Citation24 But so far there have been still limited in vivo studies on the anti-cancer effects. By using the model of P388 xenograft leukemic nude mice, we provided strong in vivo evidences that Que could promote cell apoptosis by regulating cell cycle. Bcl-2 is the founding member of the Bcl-2 family of regulator that inhibits apoptosis.Citation25,Citation26 NF-κB, a protein complex that controls transcription of DNA, is found in almost all animal cell types and involved in immune response,Citation27 cell proliferation, and cell survival.Citation28 Increased susceptibility to apoptosis caused by NF-κB defection could increase cell death. NF-κB regulates anti-apoptotic genes of TRAF1 and TRAF2; therefore, it can inhibit the activities of the caspase enzymes, which are central to most apoptotic processes.Citation28 Our results showed that Que could promote apoptosis by inhibiting the expression of NF-κBp65 and Bcl-2, and increasing Caspase-3 activity and cell apoptosis, which are consistent with the mechanistic framework generated so far.
Since its discovery from Streptomyces peucetius in the 1950s and clinical trials in the 1960s, ADM has been used as an important chemotherapeutic drug for a broad range of cancer types, such as leukemias, Hodgkin's lymphoma, bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, and multiple myeloma. However, when the cumulative dose reaches 550 mg/m2, there will be a high risk of developing cardiac side effects.Citation29 The cardiotoxicity of ADM is caused by a dose-dependent decline in mitochondrial oxidative phosphorylation.Citation30 What is more, the ROS generated by the interaction between ADM and iron will cause myofibrillar loss and cytoplasmic vacuolization. Our results indicated that Que could effectively alleviate the cardiomyocyte damages caused by ADM. We further found out Que treatment could increase the GSH-Px and SOD activities, which are the most important enzymes to clean the intracellular ROS and protect the organism from oxidative damage. Some reports pointed out that efficient antioxidant activity of Que is related to its reducing properties, such as a 3′,4′-dihydroxy(=catechol) moiety, a C4 = O keto-group, a 3-hydroxyl substituent, and a C2 = C3 double bond.Citation31 Interestingly, Que could also promote the expression of the enzymes to clean the ROS. More investigations are needed to reveal how Que triggers the signaling pathways.
In spite of the anti-cancer aspects, Que may be toxic, genotoxic, and carcinogenic. The mutagenic properties of Que have been demonstrated in a variety of bacterial and mammalian mutagenicity tests, and have been related to its quinone/quinone methide chemistry.Citation32,Citation33 There is a long way to go in terms of clinical use of Que, and future efforts are needed to estimate the biological safety of Que. Structure modification of Que might be useful to alleviate or eliminate the genotoxicity of Que.
In conclusion, by using the P388 xenograft nude mice model, our study showed that Que significantly prolonged the survival time of P388 leukemic mice by inhibiting the proliferation of white blood cells by trapping them at the S phase. Que also promoted cell apoptosis by activating Caspase-3, which is caused by suppressed expression of Bcl-2 and NF-κB. Que could also attenuate the heart damage caused by ADM through cleaning the ROS. Our study may provide strong evidences for the underlying mechanism of anti-leukemic effect of Que and sheds light on the clinical application of Que in leukemia treatment.
Acknowledgment
We wish to express our warm thanks to Fenghe (Shanghai) Information Technology Co., Ltd. Their ideas and help gave a valuable added dimension to our research.
Disclaimer statements
Contributors YH and HY participated in the design of this study, and they both performed the statistical analysis. JW carried out the study, together with YR, collected important background information, and drafted the manuscript. XS and YS conceived of this study, and participated in the design and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding The National Natural Science Foundation of China (81041045).
Conflicts of interest None.
Ethics approval All animal studies have been approved by The Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University and performed in accordance with the ethical standards.
References
- Kilari D, Venci N, Friedberg J, Bennett JM. Hemophagocytic lymphohistiocytosis masquerading as progressive chronic lymphocytic leukemia. Leuk Res Rep. 2013;2(1):4–6.
- Kodidela S, Suresh Chandra P, Dubashi B. Pharmacogenetics of methotrexate in acute lymphoblastic leukaemia: why still at the bench level? Eur J Clin Pharmacol. 2014;70(3):253–260.
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380(9859):2095–128.
- Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006;107(3):885–91.
- Gribben JG. Stem cell transplantation in chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2009;15(1):53–8.
- Laginha KM, Verwoert S, Charrois GJ, Allen TM. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin Cancer Res. 2005;11(19):6944–9.
- Fornari FA, Randolph JK, Yalowich JC, Ritke MK, Gewirtz DA. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 1994;45(4):649–56.
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17(5):421–33.
- Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer. 1967;20(3):333–53.
- Lefrak EA, Pit'ha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32(2):302–14.
- Chen J-Y, Hu R-Y, Chou H-C. Quercetin-induced cardioprotection against doxorubicin cytotoxicity. J Biomed Sci. 2013;20(1):95.
- Verschoyle RD, Steward WP, Gescher AJ. Putative cancer chemopreventive agents of dietary origin – how safe are they? Nutr Cancer. 2007;59(2):152–62.
- Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269(2):315–25.
- Nöthlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Flavonols and pancreatic cancer risk the multiethnic cohort study. Am J Epidemiol. 2007;166(8):924–31.
- Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11(4):380–1.
- Li M, He Z, Ermakova S, Zheng D, Tang F, Cho Y-Y, et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (−)− epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol Biomarkers Prev. 2007;16(3):598–605.
- Ermakova S, Choi BY, Choi HS, Kang BS, Bode AM, Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem. 2005;280(17):16882–90.
- Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chem Biol Interact. 2002;141(1):3–24.
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Ann Rev Pharmacol Toxicol. 2003;43(1):309–34.
- Rietjens IM, Boersma MG, van der Woude H, Jeurissen SM, Schutte ME, Alink GM. Flavonoids and alkenylbenzenes: mechanisms of mutagenic action and carcinogenic risk. Mutat Res FundamMol Mech Mutagen. 2005;574(1):124–38.
- van der Woude H, Alink GM, van Rossum BE, Walle K, van Steeg H, Walle T, et al. Formation of transient covalent protein and DNA adducts by quercetin in cells with and without oxidative enzyme activity. Chem Res Toxicol. 2005;18(12):1907–16.
- Neuhouser ML. Review: dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004;50(1):1–7.
- Paliwal S, Sundaram J, Mitragotri S. Induction of cancer-specific cytotoxicity towards human prostate and skin cells using quercetin and ultrasound. Br J Cancer. 2005;92(3):499–502.
- Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol. 2011;68(6):1449–57.
- Hoetelmans R. Nuclear partners of Bcl-2: Bax and PML. DNA Cell Biol. 2004;23(6):351–4.
- Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t (14; 18) translocation. Cell 1986;47(1):19–28.
- Livolsi A, Busuttil V, Imbert V, Abraham RT, Peyron JF. Tyrosine phosphorylation-dependent activation of NF-κB. Eur J Biochem. 2001;268(5):1508–15.
- Sheikh MS, Huang Y. Death receptor activation complexes: it takes two to activate TNF receptor 1. Cell Cycle. 2003;2(6):549–51.
- Minami M, Matsumoto S, Horiuchi H. Cardiovascular side-effects of modern cancer therapy. Circ J. 2010;74(9):1779–86.
- Xi L, Zhu S-G, Das A, Chen Q, Durrant D, Hobbs DC, et al. Dietary inorganic nitrate alleviates doxorubicin cardiotoxicity: mechanisms and implications. Nitric Oxide. 2012;26(4):274–84.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20(7):933–56.
- Brown J. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat Res Rev Genetic Toxicol. 1980;75(3):243–77.
- Macgregor JT, Jurd L. Mutagenicity of plant flavonoids: structural requirements for mutagenic activity in Salmonella typhimurium. Mutat Res Environ Mutagen Relat Subj. 1978;54(3):297–309.