Abstract
Objectives
Studies on experimental animals have shown that elevated oxidative stress in erythrocytes leads to the formation of autoantibodies against carbonic anhydrase (CA) and anemia. This study investigated the presence of CA I and II autoantibodies in patients with iron deficiency anemia (IDA).
Methods
Forty patients with IDA and 40 healthy controls were included in the study. Serum CA I and II autoantibody levels were analyzed using enzyme-linked immunosorbent assay. Serum malondialdehyde (MDA) levels were also measured in order to evaluate oxidative stress.
Results
CA I and II antibody titers in patients with IDA were higher than those in the controls (P = 0.005 and 0.010, respectively). A weak negative correlation was determined between anti-CA I antibody titers and ferritin, iron and mean cell volume (MCV) levels (P = 0.013, 0.042, and 0.021, respectively). Serum MDA levels were also significantly higher in the IDA group (P < 0.001). At an anti-CA I cut-off point of 0.155 absorbance unit (ABSU), sensitivity was 70% and specificity 65%. At an anti-CA II cut-off point of 0.088 ABSU, sensitivity was 60% and specificity 75%.
Discussion and conclusion
In conclusion, an immune response against CA I and II develops in IDA. CA I autoantibodies are correlated with hematological parameters used in the diagnosis of IDA and have the potential to be used in treatment.
Introduction
Iron is an essential element for all organisms. An adult will contain some 4 g of iron, approximately 75% of which is associated with hemoglobin. In addition to the transport of oxygen to tissues, it also plays an important role in energy metabolism through involvement in cytochrome structures.Citation1,Citation2 Iron deficiency is the most common nutritional problem worldwide, causing iron deficiency anemia (IDA) in approximately 500–600 million individuals.Citation3 Insufficient hemoglobin synthesis and decreased erythrocyte production occur in association with a low serum iron concentration. Mean erythrocyte life span also decreases in these patients. Erythrocyte disposition to oxidative stress increases in association with a decrease in the activities of such antioxidant enzymes as catalase and glutathione, which contain iron in their structures.Citation1,Citation4
Carbonic anhydrase (CA; EC 4.2.1.1) is a zinc enzyme that catalyzes reversible hydration of CO2. Sixteen CA isoenzymes with different tissue distributions and cellular localizations have been described in mammals.Citation5 CA I and II are cytosolic enzymes found in large quantities in erythrocytes. After hemoglobin, CA I is the most abundant protein in erythrocytes. CA II is a high-activity isoenzyme responsible for much total CA activity in several tissues.Citation6 These isozymes play physiological roles in erythrocytes, including CO2 transport, ion secretion, and pH regulation.Citation5,Citation6 Anemia and an autoimmune response to CA II have been described in superoxide dismutase 1-deficient mice.Citation7 The mechanism of antibody formation has not yet been identified, though it has been suggested that oxidative modifications of erythrocyte components in association with increased oxidative stress in erythrocytes may trigger the autoimmune process.Citation8 In some diseases, such as Sjögren's syndrome (SJS), autoimmune response against the CA II isoenzyme were reported in several studies.Citation9–Citation12 Typical symptoms like high urinary pH levels and renal tubular acidosis observed in SJS are linked to CA II autoantibodies.Citation13 In a study, in patients with autoimmune pancreatitis with tubulointerstitial nephritis, CA II antibodies were found to be elevated.Citation14 Although the mechanism of autoantibody formation has not yet been fully explained, the potential role of oxidative stress was proposed in the process.Citation7 Although studies with experimental animals have shown that oxidative stress in erythrocytes leads to anemia and represents an autoimmune response to CA II, no studies has been performed concerning the presence of CA autoantibodies in patients with iron deficiency.
The aim of this study is to investigate the presence and diagnostic value of CA autoantibodies in patients with IDA.
Materials and methods
Study population
After approval had been received from the local ethics committee of the School of Medicine, informed consent was obtained from all patients and controls. Forty patients with untreated IDA (29 females, 11 males, mean age 49.25 ± 12.83 years) were selected for this study. Description criteria for IDA were hypochromic erythrocytes with a mean corpuscular volume <80 fl, hemoglobin concentration <120 g/l, serum iron concentration <8.06 µmol/l, and serum ferritin concentration <15 µg/l. Clinical parameters in patients with IDA are listed in . Patients with other connective tissue diseases, such as systemic lupus erythematosus, systemic sclerosis, polymyositis, Behçet's disease, diabetes mellitus, alcohol abuse, and smoking were excluded. Forty healthy volunteers (29 female, 11 male, mean age 48.50 ± 10.35 years) were chosen as the control group.
Table 1. Laboratory parameters in patient with IDA
Blood samples were collected in the morning between 09.00 and 10.00 a.m. Sera samples were obtained by centrifugation at 1000 ×g for 10 minutes after clotting. These were then stored at −80°C until biochemical analyses.
Serum iron and total iron-binding capacity levels were determined using the colorimetric method (Beckman Coulter AU 5800, Japan). MCV, hemoglobin concentrations and erythrocyte numbers were measured using a Beckman Coulter LH780 (USA) device. Serum ferritin levels were measured using the chemiluminescence method (DXI 800, USA).
Enzyme-linked immunosorbent assay for serum antibody to CA I and II
Human CA I and II, electrophoretically purified from erythrocytes, was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Serum anti-CA I and II antibody titers were detected using enzyme-linked immunosorbent assay following a previously described method.Citation11 The absorbance was read at 480 nm. Control wells that were not coated with CA II or CA I were also used for enzyme-linked immunosorbent assay of each serum studied. All assays were performed in duplicate, and the specific binding of serum anti-body to CA II or CA I was calculated as follows: the average absorbance of the antigen coated wells minus the average absorbance of control wells (Specific binding = A coated-A control).
Samples from a positive IDA patient and a control subject were used for determination of intra-assay coefficient of variation. For anti-CA I and II antibody assay, the intra-assay coefficient of variation was 6.7% (absorbance at 480 nm = 0.371) and 4.2% (absorbance at 480 nm = 0.260) (n = 5) in the IDA patient and 5.8% absorbance at 480 nm = 0.141) and 3.6% (absorbance at 480 nm = 0.072) (n = 5) in the control, respectively.
Malondialdehyde assays
Lipid peroxidation in serum samples was determined as malondialdehyde (MDA) concentration using the method previously described by Yagi.Citation15 Tetramethoxypropane was used as a standard, and MDA levels were calculated as nmol/ml.
Statistical analysis
The Kolmogorov-Smirnov test was used to test for normal distribution of continuous variables. Data characterized by a normal distribution were expressed as mean values followed by standard deviation. Parameters without such a distribution were expressed as median with interquartile range. Student test (normal distribution) or Mann–Whitney test (non-normal distribution) were used to compare to differences between the IDA and control groups. Spearman's correlation coefficient analysis was used to examine the relationship between the serum anti-CA I and II antibody titers and other laboratory parameters. The investigation for a diagnostic cutoff value was based on receiver–operating characteristic (ROC) curves. P < 0.05 was considered statistically significant.
Results
Mean values of laboratory findings are listed in . Anti-CA I and II antibody titers in the IDA and control groups are shown in A and B, respectively. CA I antibody titers in patients with IDA (0.194 ± 0.073) were significantly higher than those in the controls (0.151 ± 0.058) (P = 0.005) (). A weak negative correlation was determined between anti-CA I antibody titers and ferritin, iron, and MCV levels (r = −0.354, P = 0.013, r = −0.277, P = 0.042, and r = −0.324, P = 0.021, respectively) ().
Figure 1. (A) Anti-CA I antibodies in sera from patients with iron deficiency anemia (IDA) and healthy controls. (B) Anti-CA II antibodies in sera from patients with IDA and healthy controls.
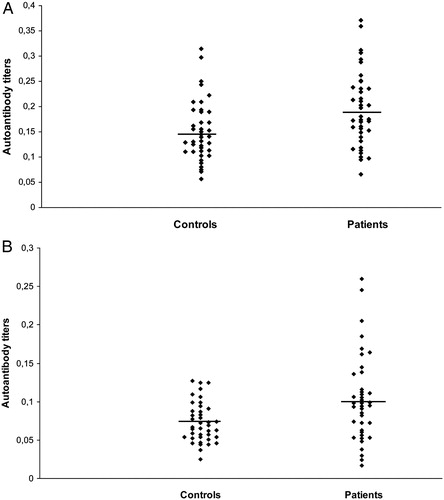
Table 2. The mean values of laboratory findings in study groups
Table 3. Correlations between anti-CA antibodies and other laboratory findings in IDA patients
The mean absorbance value of anti-CA II antibody titers for the control subjects was 0.073 ± 0.025. The mean absorbance value in the IDA group (0.102 ± 0.055) was significantly higher compared with that of the control group (P = 0.009) (). No correlation was determined between CA II antibody titers and other parameters (). Serum MDA levels were significantly higher in the IDA group (3.31 ± 3.32) compared with the control group (1.68 ± 0.78), (P < 0.001) ().
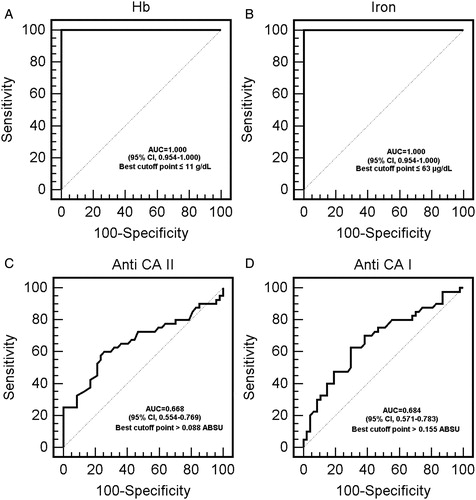
For evaluation of laboratory findings using the ROC curve method, the optimum diagnostic cut-off points were anti-CA I antibody 0.155 and anti-CAII antibody 0.088. The specified cut-off points and the sensitivity, specificity, and the area underneath the ROC curve (AUC) for those cut-off points are shown in and A–D.
Table 4. The optimum diagnostic Hb, iron, anti-CA I and anti-CA II levels cut-off point, sensitivity, and specificity according to the receiver operator characteristic curve
Discussion
Our study is the first to determine an autoimmune response to CA I and II in patients with IDA. Both CA I and CA II antibody titers were higher in the patients than in the controls (P < 0.05) ( and A and B). By the ROC analysis, the area under the curve to predict IDA was 0.684 for anti-CA I, 0.668 for anti-CA II, 1.000 for hemoglobin, and 1.000 for iron (, A–D). At an anti-CA I cut-off point of 0.155 ABSU, sensitivity was 70% and specificity 65%. At an anti-CA II cut-off point of 0.088 ABSU, sensitivity was 60% and specificity 75%. A cut-off value of 11 g/dl for hemoglobin was associated with 100% sensitivity and 100% specificity. A cut-off value of 63 µg/dl for iron was associated with 100% sensitivity and 100% specificity. Although sensitivity and specificity of anti CA I and II levels were not found fairly close to the hemoglobin and iron values, we think that these markers may support – the diagnosis of IDA. These antibodies have previously been demonstrated in autoimmune-based diseases, such as rheumatoid arthritis, SJS, and primary biliary cirrhosis,Citation10,Citation12,Citation16 but the mechanism involved in antibody formation was not identified. In a study of SOD 1 knock-out mice, oxidative stress in erythrocytes was shown to trigger CA II autoantibody formation.Citation7
Figure 2. (A) Receiver operator characteristic (ROC) curve analysis of iron deficiency anemia (IDA) Hb values. (B) ROC curve analysis of IDA iron values. (C) ROC curve analysis of IDA anti CA II values. (D) ROC curve analysis of IDA anti-CA II values.
CA I is mainly synthesized in erythrocytes, and is the most abundant protein in erythrocytes after hemoglobin. Although it is five to six times more abundant than CA II, it is responsible for 50% of CA activity.Citation6 Although its expression has been observed to increase in various forms of anemia, expression decreases in hemolytic anemia. On that basis, it has been suggested that CA I can be used as a marker for hemolytic anemia.Citation17 CA I autoantibodies have been observed to be simultaneously or independently high together with CA II autoantibodies in several autoimmune diseases.Citation15,Citation18 Anti-CA I antibodies were also high in our patients with IDA ( and A). The negative correlation with MCV, ferritin, and iron values () suggests that the isoenzyme may be involved in the pathogenesis of IDA.
CA II is an abundant, cytosolic isoenzyme present in almost every tissue,Citation6 immunization in mice results in autoimmune sialadenitis.Citation19 Although the mechanisms are uncertain, oxidative stress has been reported to be potentially significant in the formation of these autoantibodies.Citation7,Citation8,Citation11,Citation12 In our study, the possible mechanisms by which autoantibodies against CA I and II form in patients with IDA may be summarized as follows: lipid peroxidation end products 4-hydroxy-2-nonenal (HNE) and MDA modify proteins and alter their antigenic properties.Citation20 One study with erythrocytes demonstrated that CA II was the first target of HNE.Citation21 CA I and II present in erythrocytes may acquire an antigenic property because of similar modifications. In this study, serum MDA values were high in patients with IDA, although no correlation was observed between antibody titers and MDA. This may be due to serum MDA levels not completely reflecting erythrocyte MDA levels. Apoptotic bodies have also been reported to be important in the formation of antibodies against intracellular proteins.Citation22 Erythrocyte destruction increases eryptosis in patients with IDA.Citation23 CA I and II autoantibodies may have formed against another antigenic CA isozyme because of molecular mimicry.
It is difficult to be definitive regarding the role of CA autoantibodies in the pathology of IDA, although these antibodies have been shown to cause enzyme inhibition.Citation24 Depending on our present correlations, one can speculate that inhibition of these isoenzymes may occur as a result of these autoantibodies entering the erythrocyte and causing changes in CO2 transport, intracellular pH, the oxygen–hemoglobin relationship and transport metabolons. This may lead to a further deepening of iron anemia symptoms.
Our results demonstrates that CA I and CA II antibodies may be useful markers for clinicians in distinguishing IDA as hemoglobin, iron, MCV, and ferritin.
In conclusion, CA I and II autoantibody titers were significantly higher in patients with IDA than in the controls. These autoantibodies are correlated with important parameters in the diagnosis and monitoring of IDA, such as ferritin, MCV, and iron. Optimum diagnostic cut-off point, AUC and sensitivity and specificity values for hemoglobin, iron, anti-CA I and anti-CA II levels are given in . The table shows lower sensitivity and specificity for anti-CA I and anti-CA II than hemoglobin and iron. On the basis of the ROC curve and our results, however, we think that anti-CA I and anti-CA II measurement may be useful in the diagnosis of IDA for clinicians. These may therefore be a promising marker for IDA. There is also a weak negative correlation was determined between anti-CA I antibody titers and ferritin, iron and MCV levels. Further follow-up studies are needed to investigate the prognostic role of anti-CA I and anti-CA II in patients with IDA.
Limitations
The major limitation of the study is the relatively small number of patients and controls included. However, due to the novel idea of using anti-CA II antibody and MDA as a potentially new diagnostic and prognostic biomarker, our study can be considered a pioneering work in the field and can serve as a basis for further comprehensive studies.
Disclaimer statements
Contributors AM and AA are guarantors of the integrity of the entire study, and were involved in study concepts and design, and manuscript preparation. AM, AA, and NE were responsible for literature research. Clinical studies were carried out by MS and NE. Experimental studies/data analysis were carried out by AM, AS, and DUA. AA was involved in Statistical analysis. Manuscript editing was done by AA, AS, AM, and DUA.
Funding This research was partly funded by the Karadeniz Technical University Research Fund (Project No. 8682).
Conflicts of interest None.
Ethics approval The study was conducted in accordance with the Helsinki declaration and was approved by the local research ethics committee (no. 2013/12).
References
- Nagababu E, Gulyani S, Earley CJ, Cutler RG, Mattson MP, Rifkind JM. Iron-deficiency anaemia enhances red blood cell oxidative stress. Free Radic Res. 2008;42:824–9.
- McDermid JM, Lönnerdal B. Iron. Adv Nutr. 2012;3:532–3.
- Yoo JH, Maeng HY, Sun YK, Kim YA, Park DW, Park TS, et al. Oxidative status in iron-deficiency anemia. J Clin Lab Anal. 2009;23:319–23.
- Madhikarmi NL, Murthy KR. Antioxidant enzymes and oxidative stress in the erythrocytes of iron deficiency anemic patients supplemented with vitamin. Iran Biomed J. 2014;18:82–7.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7:168–81.
- Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401.
- Iuchi Y, Okada F, Onuma K, Onoda T, Asao H, Kobayashi M, et al. Elevated oxidative stress in erythrocytes due to SOD1 deficiency causes anemia and triggers autoantibody production. Biochem J. 2007;402:219–27.
- Iuchi Y, Okada F, Takamiya R, Kibe N, Tsunoda S, Nakajima O, et al. Rescue of anaemia and autoimmune responses in SOD1-deficient mice by transgenic expression of human SOD1 in erythrocytes. Biochem J. 2009;422:313–20.
- Inagaki Y, Jinno-Yoshida Y, Hamasaki Y, Ueki H. A novel autoantibody reactive with carbonic anhydrase in sera from patients with systemic lupus erythematosus and Sjögren's syndrome. J Dermatol Sci. 1991;2(3):147–54.
- Invernizzi P, Battezzati PM, Crosignani A, Zermiani P, Bignotto M, Del Papa N, et al. Antibody to carbonic anhydrase II is present in primary biliary cirrhosis (PBC) irrespective of antimitochondrial antibody status. Clin Exp Immunol. 1998;144:448–54.
- Alver A, Menteşe A, Karahan SC, Erem C, Keha EE, Arikan MK, et al. Increased serum anti-carbonic anhydrase II antibodies in patients with Graves’ Disease. Exp Clin Endocrinol. 2007;115:287–91.
- Alver A, Şentürk A, Çakirbay H, Menteşe A, Gökmen F, Keha EE, et al. Carbonic anhydrase II autoantibody and oxidative stress in rheumatoid arthritis. Clin Biochem. 2011;44:1385–9.
- Pertovaara M, Bootorabi F, Kuuslahti M, Pasternack A, Parkkila S. Novel carbonic anhydrase autoantibodies and renal manifestations in patients with primary Sjogren's syndrome. Rheumatology (Oxford) 2011;50(8):1453–7.
- Nishi H, Tojo A, Onozato ML, Jimbo R, Nangaku M, Uozaki H, et al. Anti-carbonic anhydrase II antibody in autoimmune pancreatitis and tubulointerstitial nephritis. Nephrol Dial Transplant. 2007;22(4):1273–5.
- Yagi K. Lipid peroxides and related radicals in clinical medicine. Adv Exp Med Biol. 1994;366:1–15.
- Kino-Ohsaki J, Nishimori I, Morita M, Okazaki K, Yamamoto Y, Onishi S, et al. Serum antibodies to carbonic anhydrase I and II in patients with idiopathic chronic pancreatitis and Sjögren's syndrome. Gastroenterology 1996;110:159–1586.
- Kuo WH, Yang SF, Hsieh YS, Tsai CS, Hwang WL, Chu SC. Differential expression of carbonic anhydrase isoenzymes in various types of anemia. Clin Chim Acta. 2005;351:79–86.
- Itoh Y, Reichlin M. Antibodies to carbonic anhydrase in systemic lupus erythematosus and other rheumatic disease. Arthritis Rheum. 1992;35:73–82.
- Nishimori I, Bratanova T, Toshkov I, Caffrey T, Mogaki M, Shibata Y, et al. Induction of experimental autoimmune sialoadenitis by immunization of PL/J mice with carbonic anhydrase II. J Immunol. 154:4865–73.
- Toyoda K, Nagae R, Akagawa M, Ishino K, Shibata T, Ito S, et al. Protein-bound 4-hydroxy-2-nonenal: an endogenous triggering antigen of antI-DNA response. J Biol Chem. 2007;282:25769–78.
- Uchida K, Hasui Y, Osawa T. Covalent attachment of 4-hydroxy-2-nonenal to erythrocyte proteins. J Biochem. 1997;22:1246–51.
- Racanelli V, Prete M, Musaraj G, Dammacco F, Perosa F. Autoantibodies to intracellular antigens: generation and pathogenetic role. Autoimmun Rev. 2011;10:503–8.
- Lang F, Lang E, Föller M. Physiology and pathophysiology of eryptosis. Transfus Med Hemother. 2012;39:308–14.
- Botrè F, Botrè C, Podestà E, Podda M, Invernizzi P. () Effect of anti-carbonic anhydrase antibodies on carbonic anhydrase I and II. Clin Chem. 2003;49:1221–3.
