Abstract
Background
Glucocorticoids are essential in protocols of therapy of acute lymphoblastic leukemia (ALL).
Objectives
To assess the incidence, severity, morbidity, and risk factors of hypothalamic-pituitary-adrenal axis (HPA) suppression in children with ALL, and the time course of recovery.
Design
Forty standard risk ALL children treated in the Pediatric Hematology/Oncology Unit, Ain-Shams University, Egypt, were classified into dexamethasone (DXM) group: 20 patients on children cancer group protocol and prednisone (PDN) group: 20 patients on modified Berlin-Frankfurt-Muenster (BFM) study group 90 protocol. Patients were followed clinically and by laboratory assessment of morning s.ACTH, basal and after low-dose adrenocorticotrophic hormone stimulation test of cortisol and DHEAS, at diagnosis and every 2 weeks till adrenal recovery.
Results
HPA recovery was earlier in PDN than DXM group (P < 0.05). In induction phases 1 and 2: 65 and 75% of PDN group recovered on week 2, while 45 and 50% of DXM group recovered in week 4. Adrenal recovery was predicted 2 weeks earlier by normalized s.DHEAS. Children below 5 years of age had earlier recovery in PDN group (P = 0.04), no age effect in DXM group.
Conclusion
Adrenal suppression is an inevitable consequence of ALL therapy. Monitoring of cortisol levels and steroid coverage during stress is recommended, and gradual steroid tapering is suggested.
Introduction
Children with acute lymphoblastic leukemia (ALL) usually receive multidrug agents during their course of chemotherapy. Glucocorticoids represent the cornerstone in the different phases of different treatment protocols of ALL.Citation1 High-dose glucocorticoid therapy results in suppression of hypothalamo-pituitary-adrenal axis (HPA) and increased risk of adrenal insufficiency. The adrenal insufficiency observed in ALL is rarely clinically manifested, but it is more commonly subtle and manifests as impaired cortisol response to stressful stimuli.Citation2 The time course of recovery of HPA after glucocorticoid therapy in children treated for ALL shows marked interindividual variations.Citation3
The diagnosis of central adrenal insufficiency is difficult. Insulin-induced hypoglycemia test is considered to be the gold standard test; however, it has many limitations of use in children.Citation4 The standard dose adrenocorticotrophic hormone (ACTH) test is an alternative test in diagnosis of central adrenal insufficiency.Citation5 Moreover, the low-dose ACTH (LD-ACTH) test, where 1 μg of ACTH (instead of 250 μg) is given as the test dose, is more sensitive and more specific.Citation6 Serum dehydroepiandrostenedione-sulphate (DHEA-S) level is a reliable and sensitive alternative approach in evaluating adrenal function.Citation7
The aim of this study was to assess the incidence, severity, morbidity, and risk factors of HPA suppression in children with ALL, and the time course of recovery, using two different corticosteroids containing protocols.
Subjects and methods
Subjects
The study is a prospective study, done from January 2005 till June 2008, in the Pediatric Hematology/Oncology Unit, Children's Hospital, Ain Shams University, Cairo, Egypt. Prior to 2006, ALL patients were on BFM 90 protocol; and since 2006, treatment was changed to children cancer group (CCG)-based protocols. Forty newly diagnosed standard risk ALL children were consecutively enrolled in the study, after obtaining written parental consent. The study was approved by faculty ethical committee. We excluded from our study patients below the age of 12 months, patients whose parents refused to allow them to participate in the study, patients with previous adrenal disorders, and patients with history of steroids intake before diagnosis of leukemia.
Study protocol
The 40 ALL patients were classified into two groups according to the types of corticosteroid they received as per their protocol of therapy:
| 1. | The dexamethasone (DXM) group comprised 20 newly diagnosed ALL children who followed the CCG-1991 protocol. They received DXM in induction phases 1 and 2. They were 7 boys and 13 girls. | ||||
| 2. | The prednisone (PDN) group comprised 20 newly diagnosed ALL children who received PDN during their induction phases 1 and 2 of therapy according to modified BFM-1990 protocol. They were 9 girls and 11 boys. | ||||
Treatment given for leukemia patients was as follows:
Induction of remission
| • | In the DXM arm, patients received treatment according to the CCG-1991 protocol. All shared pulsed vincristine (VCR) 1.5 mg/m² (days 0, 7, 14 and 21), DXM 6 mg/m² for 28 consecutive days, and l-asparginase 6000 IU/m² for nine doses every other day. | ||||
| • | In the PDN arm, all subjects received PDN 60 mg/m² for 28 successive days, weekly pulses of VCR 1.5 mg/m², daunomycin (DNM) 30 mg/m² on days 8, 15, 22, and 29, and 12 alternating doses of l-asparginase at 10 000 IU/m² according to modified BFM-oriented schedule. | ||||
All patients received age-adjusted central nervous system (CNS) prophylaxis on days 0, 14, and 21.
Re-induction phase (induction 2)
The 40 patients received three weeks of full dose corticosteroids (60 mg/m²/day PDN versus 6 mg/m²/day DXM) and 4 weekly pulsed doses of VCR 1.5 mg/m²/day, DNM 25–30 mg/m² and alternating l-asparginase at 10 000 IU/m² for four doses in BFM protocols or at 10 000 IU/m² for 9–12 doses in CCG protocol.
Data and sample collection
All patients were subjected to the following:
(a) Full history taking laying stress on previous history of steroid intake for any other disease, family or past history of hypothalamic, pituitary or adrenal gland-related disorders.
(b) Complete data records of drugs taken by the patient including corticosteroids (type of preparation, dose, and timing); other chemotherapeutic agents, other drugs used as prophylaxis and/or therapy against fungal and viral infections.
(c) Careful assessment of clinical manifestations of steroid withdrawal syndrome including subjective symptoms (e.g. anorexia, nausea, arthralgia, myalgia, abdominal pain, and low performance) and objective signs (e.g. weight loss, hypotension, hypoglycemia, fever with absolute neutrophil count >500/mm³).
(d) The duration and frequency of hospitalization for septic episodes, number of days with neutropenia >500/mm³ were recorded. The infectious events were graded from mild to severe according to whether febrile neutropenic patients were managed as outpatient or needed hospitalization for antimicrobial coverage. Life-threatening toxic events associated with adrenal decline calling for life-saving stress steroid doses were graded as the most severe and were also recorded. We recorded data about whether fluconazole (FCZ) was used or not in the febrile event and the dose given (<10 mg/kg/day or more).
Biochemical measurements
All patients included in the study were subjected to the following laboratory investigations:
| • | Complete blood picture with differential blood count initially and during phases of therapy, bone marrow aspirate, cytology, and immunophenotyping, lumbar puncture with cerebrospinal fluid (CSF) cytology, complete metabolic profile including liver and kidney functions; serum sodium, potassium, and random blood sugar; done initially and followed up during various phases of therapy. | ||||
| • | Adrenal function testing using the LD-ACTH test. Testing was done between 8 and 11 AM in the morning as follows: one vial of 250 mcg of tetracosactrin (Synacthen, Novartis, Basel, Switzerland) was diluted in sterile saline solution to a concentration of 1 mcg/ml, filtered in plastic syringes and stored at 4°C. The blood samples were obtained from a wide bore peripheral venous line immediately before administration of intravenous 1 mcg/1 ml of tetracosactrin to determine the basal morning and the stimulated values of s.ACTH, cortisol, DHEAS (after 30 minutes of injection). The LD-ACTH test was done at the following phases: at diagnosis (before starting steroid therapy), post-steroid tests done on weeks 0, 2, and 4 after the last steroid dose, and every two weeks and during subsequent febrile neutropenic episodes till HPA axis recovery, a normal response was defined as a stimulated serum cortisol >18 μg/dl. For children with lower peak values, an increment from basal line of at least 6 μg/dl was considered a normal response. Also we evaluated the value of DHEAS in assessing HPA axis suppression and predicting adrenal recovery. | ||||
Hormonal assay
All blood samples were frozen and stored for analysis in one batch after termination of the study. Serum cortisol was measured by using direct chemiluminescent technology. The 95% morning cortisol range is 4–27 mcg/dl. The intra-assay coefficients of variation were 2.4 and 10.3% at serum concentrations of 8.4 mcg/dl and 92.2 mcg/dl, respectively. The interassay coefficients of variation were 12 and 8% at serum concentrations of 5 and 15 mcg/dl, respectively. The ACTH range was 7.6–66 pg/ml. The DHEAS range was 0.4–2.1 mcg/ml for girls and 0.59–2.96 mcg/ml for boys.
Statistical analysis
The data were coded, entered, and processed on an IBM-PC compatible computer using SPSS (version 15). Normally distributed variables were reported as mean ± SD. Skewed data were reported as median ± range. Independent t tests were used to compare normally distributed continuous variables. The χ² test was used for analysis of categorical data. Standard deviation scores (z scores) for height and weight were determined from age- and sex-specific reference values. The Wilcoxon signed-rank test was performed in comparison between infection on inductions 1 and 2. The McNemar test was used to determine whether the initial response rate equals the final response rate.
Results
Clinical and demographic
Forty patients were recruited during the study period. The male : female ratio was 7 : 13 in the DXM group, compared with 11 : 9 in the PDN group.
Asymptomatic adrenal insufficiency was detected in 27.5% of patients before treatment. By comparing the two groups of steroids therapy according to the timing of occurrence of symptoms in inductions 1 and 2, it was found that symptoms and signs of steroid withdrawal syndrome developed in 50% of PDN group and 75% of DEXA group after induction phases I and II; they occurred on days 1–3 and on days 4–9 following DXM and PDN tapering, respectively.
Comparing DXM and PDN arms as regards the mean weeks of adrenal recovery revealed that, for patients on DXM the mean week at which HPA axis recovered (7.2 ± 4.5 SD) was the same in inductions 1 and 2, (P < 0.99); while patients on PDN recovered at earlier time in induction 2 (3 ± 1.89 SD) than induction 1 (4.8 ± 4.7 SD) with P = 0.04 as shown in .
Table 1. Comparison between the two groups of steroid therapy as regards the mean weeks of recovery in inductions 1 and 2
Hormonal assay
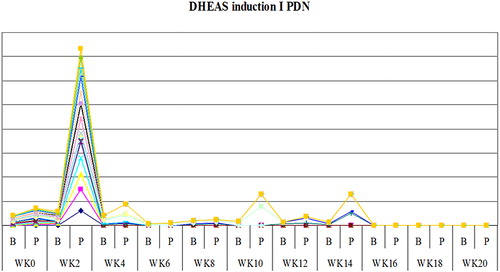
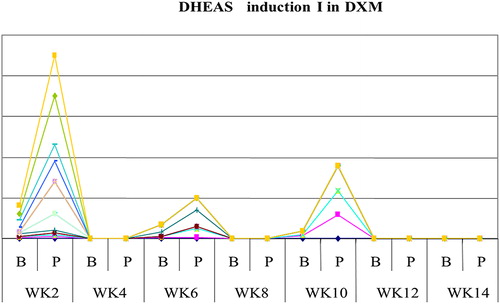
The study showed that serum cortisol and ACTH returned back to normal in most patients on DXM around week 4 both in inductions 1 and 2, as shown in and , and patients on PDN around week 2 ( and ). Also the study showed that serum DHEAS levels returned back to normal in 75 and 45% of DXM group (in inductions 1 and 2, respectively) and in 85–90% of patients on PDN two weeks before complete adrenal recovery (in inductions 1 and 2).
Figure 1. The mean weeks of recovery of adrenocorticotrophic hormone (ACTH) to normal in dexamethasone (DXM) group in induction 1.
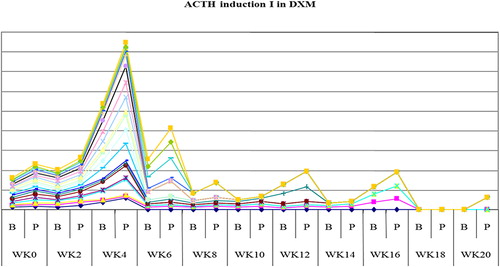
Figure 2. The mean weeks of recovery of cortisol to normal in dexamethasone (DXM) group in induction 1.
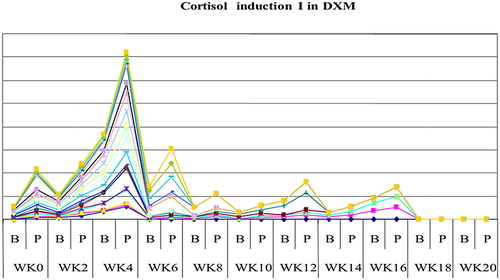
Figure 3. The mean weeks of recovery of adrenocorticotrophic hormone (ACTH) to normal in prednisone (PDN) group in induction 1.
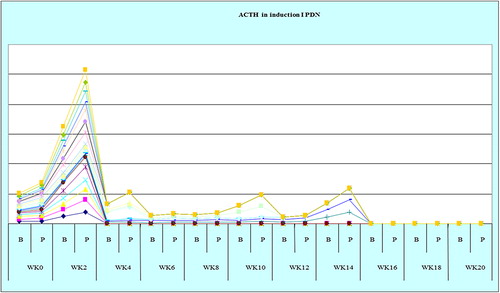
Figure 4. The mean weeks of recovery of cortisol to normal in prednisone (PDN) group in induction 1.
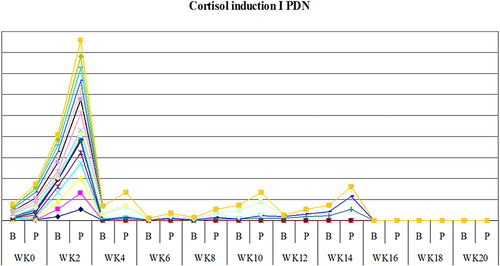
A statistically significant difference was found between the two groups of steroids as regards weeks needed for adrenal recovery, P < 0.05 (inductions 1 and 2): in the DXM group, 45–50% of the patients recovered at week 4, 20%–25% at week 6, and the rest recovered later between weeks 8 and 20 following DXM tapering in inductions 1 and 2. In the PDN group, 65–75% of patients recovered at week 2, 5–20% between weeks 4 and 6 and the rest of patients recovered later between weeks 8 and 20 after PDN tapering in inductions 1 and 2 as shown in and .
Figure 6. The mean weeks of recovery of s.DHEAS to normal in dexamethasone (DXM) group in induction 1.
Comparing between the two groups of steroids treatment as regards the relation between the time of recovery and the grading of intercurrent infections they were exposed to between inductions 1 and 2 after the post-steroid weaning period, we found that increasing numbers of intercurrent infections were associated with late recovery in inductions 1 and 2 in both PDN (P = 0.005) and DXM groups (P = 0.002).
Our results showed that 11 of 40 (27.5%) of the patients had initial adrenal suppression before starting ALL treatment as shown in . Also patients who had adrenal suppression before starting steroid therapy, showed later adrenal recovery than patients who had normal adrenal functions before starting the treatment.
Table 2. Comparison between the two groups of patients as regards the presence of initial adrenal suppression before starting steroid therapy in induction phase 1 or 2
Moreover, there was no statistical significant difference between the two groups of steroid treatment as regards the distribution of grading of infections they were exposed to during post steroid tapering in inductions 1 and 2 (P > 0.05).
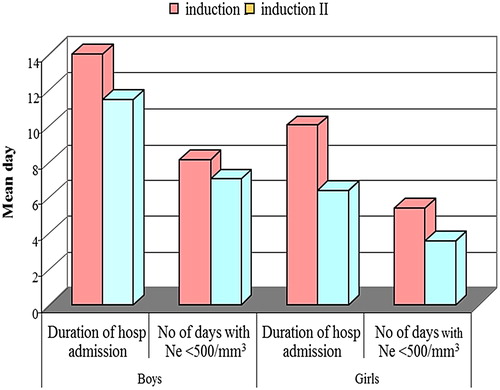
There was no statistically significant difference between DXM and PDN groups as regards the mean duration of hospitalization and mean duration of absolute neutropenia <500/mm³ following steroid tapering in inductions 1 and 2 as shown in .
Sex difference
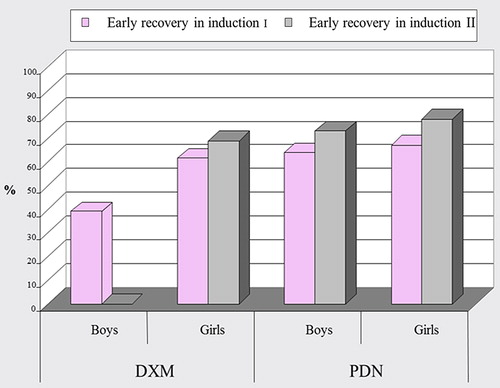
In the DXM group, boys and girls recovered at the same time after DXM tapering in induction 1, but boys showed a statistically significant late recovery than girls in induction 2, while in the PDN group, boys and girls recovered at the same time after PDN tapering in inductions 1 and 2 as shown in .
Figure 8. Comparison between boys and girls as regards the timing of adrenal recovery after tapering of dexamethasone (DXM) and prednisone (PDN) in inductions 1 and 2.
Comparing between boys and girls in DXM and PDN group as regards the grading of infection they were subjected to during poststeroid weaning period in inductions 1 and 2, we found that in the DXM group: Boys were subjected to more severe infections than girls in induction 2, while, in induction 1, they were equally subjected to infections with different grading.
In the PDN group: There was no statistical significant difference between boys and girls as regards the grading of infection, P > 0.05 in inductions 1 and 2.
In induction 1, there was no statistical significant difference between boys and girls as regards the mean duration of hospital admission and mean number of days with absolute neutropenia. Boys showed a longer duration with neutropenia <500/mm³ than girls but this didn't reach a significant level. In induction 2, boys showed a statistically significant longer duration of hospital admission and a higher mean number of days with absolute neutrophil count <500/mm³ in comparison to girls (P < 0.05), . There was no significant difference between the two groups of treatment as regards the distribution of sex, P > 0.05.
Age difference
In the DXM arm, cases aged more than 5 years showed no statistically significant difference than cases aged <5 years as regards the time of adrenal recovery. In the PDN arm, cases aged more than 5 years showed a statistically significant late recovery (P = 0.004) than cases aged <5 years.
In the DXM group, 80% of patients received FCZ in inductions 1 and 2, while in the PDN group, 55 and 35% of patients received FCZ in inductions 1 and 2, respectively.
Results showed that 50% of the patients in the two groups received FCZ at doses ≥10 mg/kg/day during their febrile neutropenic events in inductions 1 and 2.
Patients who received FCZ at dose ≥300 mg/d had showed a statistically significant later adrenal recovery than those who received cumulative dose of FCZ <300 mg/day.
Cases with added DNM showed a statistically significant prolonged neutropenia with absolute neutrophil count <500/mm³ than those who did not receive DNM during induction (P < 0.05), with no effect on the mean duration of hospitalization.
Results showed that 60% of patients in both the DXM and PDN group developed steroid-induced hypertension in induction 1 with blood pressure >90th percentile that necessitated antihypertensive therapy, while in induction 2, 50% of patients in the DXM group, and 75% of patients in the PDN group developed hypertension, the blood pressures in all patients returned back to normal after tapering of steroids. Most of the hypertensive children had positive family history of hypertension.
Results showed that 15% (6 / 40) and 12.5% (5 / 40) of the patients in the study developed overt hyperglycemia with either postprandial blood sugar >280 mg/dl or RBS >200 mg/dl on ≥2 glucose determinations in inductions 1 and 2, respectively. Five of them were on DXM and only one was on PDN. They were the same subjects in both phases. In the DXM group, 20% (4 / 20) in induction 1 and 20% (4 / 20) in induction 2 had family history of diabetes mellitus (DM). While in the PDN group, 5% (1 / 20) in induction 1 and the same in induction 2 had family history of DM. Plasma insulin and C-peptide levels were normal in all patients. In all patients with hyperglycemia, there were no concurrent l-asparaginase-induced acute pancreatitis and diabetic ketoacidosis did not occur. All of them were equally subjected to infections and needed hospital admission for antibiotic and FCZ coverage for about 10 days during inductions 1 and 2. They all received subcutaneous insulin therapy (NPH/regular) till the inductions (1 and 2) have ended and steroids were tapered.
Discussion
Cortisol (hydrocortisone), the major glucocorticoid secreted from the adrenal cortex, is essential for life.Citation8 Cortisol production in children and adults is approximately 6–8 mg/m²/day, with mean early morning plasma level of 7–22 μg/dl. During periods of stress, cortisol levels rise up to 15 times the normal level.Citation9
Cortisol secretion is regulated by corticotrophin (ACTH) secreted from the anterior pituitary gland, which, in turn, is controlled by corticotrophin-releasing hormone secreted by the hypothalamus. These glands constitute the HPA axis and their respective hormones form a classic negative feedback loop. After prolonged exposure to high doses of glucocorticoids, recovery of the adrenal cortex lags behind the pituitary, so that, basal corticotrophin levels are increased before the adrenal cortex is capable of responding. That's why careful monitoring of complete restoration of the HPA axis is important especially during stressful events. Because of their profound lympholytic properties, glucocorticoids have played a major role in the treatment of childhood ALL.Citation10
Prolonged exposure of ALL children to supraphysiologic doses of corticosteroids is usually associated with many adverse systemic effects. One of the most worrisome and treatable of these is the suppression of HPA axis and subsequent adrenal atrophy.Citation11
The cortisol response to ACTH stimulation is usually determined by the LD-ACTH test (1 μg ACTH) is considered the most sensitive (94–100%) and specific (88–100%) tool that can even detect mild degrees of adrenal impairment.Citation12
One of the most important clinical alarms for laboratory assessment of adrenal decline is detection of the so-called steroid withdrawal syndrome.Citation13 In our study, 50–75% of ALL children on DXM and PDN developed symptoms and signs of steroid withdrawal syndrome. This agrees with Saracco et al.Citation14 Most of the DXM patients (95%) symptomatized early in their first 3 days of tapering in comparison to 50% of those on PDN whose symptoms occurred by days 4–9. Symptoms were more frequent, more severe, more disabling and occurred earlier in the DXM group. Saracco et al.Citation14 explained this different behavior partially by the different ACTH suppressive effects of DXM and PDN. The former having 80 times stronger ACTH suppressing effect than PDN.
This study revealed a significant difference in the time course of recovery of the HPA axis between ALL children receiving DXM and PDN. Early recovery occurred at week 2 in 65–75% of patients on PDN in inductions 1 and 2, while in the DXM group, 45–50% of children recovered at week 4 in inductions 1 and 2. Felner et al.Citation11 found adrenal suppression lasting 4–8 weeks in 30% of children who received 4 weeks of DXM during induction, Petersen et al.Citation15 found suppression lasting 2.5–8 months in 41% of patients who received 5 weeks PDN in induction and 3 weeks DXM in re-induction phases of treatment. In 2004, Mahachoklertwattana et al.Citation16 used the LD-ACTH test and had results similar to our study. They found adrenal suppression two weeks after stopping steroids in 46% 0f children, more frequently in children older than 5 years.
DHEA and DHEAS are the most abundant steroids in the circulation and are corticotrophin-dependent androgens.Citation17 The half-life of DHEAS is 10–20 hours which is substantially longer than that of cortisol (2 hours). Thus, the diurnal changes in serum DHEAS concentrations are minor compared with those of cortisol, which has fluctuating concentrations depending on the exogenous stress and time of day.Citation17 The results of this study revealed that most children in both groups had normal DHEAS response to ACTH before complete restoration of HPA axis (i.e. before normalized basal and stimulated cortisol level). So, DHEA and DHEAS seem to be more sensitive than cortisol to corticotrophin stimulation. This was explained by the peculiar sensitivity of the reticular adrenal zone to extremely low ACTH dose. This agrees with study of Nasrallah and ArafaCitation18 in 2003, who used DHEAS to predict HPA axis function in patients with large pituitary adenomas. Their results agreed with the fact observed in the present study that DHEAS could be used as an early indicator of adrenal recovery.
In our study, children were equally subjected to febrile neutropenic episodes with no statistical difference in the mean duration of hospitalization or duration of absolute neutropenia regardless of the type of steroids, and there were no difference in the grading of infections they suffered from.
In the study of Saracco et al.Citation14 as well as in this study, there was no significant difference in infectious complications between the two groups of steroids. However, the infectious-related complications were significantly more evident and associated with more delay in adrenal recovery in DXM group after induction 2. At this point other factors could be added such as, higher frequency of intercurrent infection, use of DNM and higher doses of FCZ and so DXM cannot be accused as the only cause of delay in axis recovery.
In spite of all DXM-related septic complications in ALL during induction phase of therapy, its potential benefits over PDN could not be neglected. It has longer half-life with prolonged duration of action, higher trough levels, steady state concentrations with higher fraction of plasma DXM available for tissues and for lymphoblasts with medullary and CSF penetration with lower incidence of CNS leukemia.Citation19
In this study, 80% of patients on DXM and 35–55% of patients on PDN received FCZ for febrile neutropenia during their post-steroid tapering periods in inductions 1 and 2. 50% of these children received FCZ at doses ≥10 mg/kg/day (≥300 mg/day). This sector showed significant delay in adrenal recovery than those who had lower doses of FCZ. Shibata et al.Citation20 reported reversible adrenal suppression with inadequate ACTH response following FCZ at cumulative doses 200–800 mg/day in an adult multiple myeloma patient who received high dose FCZ and high-dose cyclophosphamide for peripheral blood stem cell harvest which then returned after FCZ discontinuation.
DNM is an important chemotherapeutic agent that is basically used in induction and re-induction phases of the BFM-oriented regimens and in high-risk members of CCG protocol. DNM is closely related to septic responses during induction phases. In this study, it was observed that addition of DNM to induction 1 in high-risk patients of CCG protocol phases was significantly associated with prolongation in duration of absolute neutropenia. This was in agreement with Balgaumi et al.,Citation21 who found that addition of DNM has augmented the DXM-related infectious toxicities compared with PDN-related infectious toxicities.
Considering the age factor and its relation to time course of HPA axis recovery, this study revealed that ALL children in the PDN group aged <5 years showed significant earlier recovery than those ≥5 years. This is similar to Mahachoklertwattana,Citation16 who explained this difference by the fact that it could be due to difference in sensitivity of the HPA axis in response to negative feedback of exogenous corticosteroids.
As regards weaning of the replacement hydrocortisone stress doses, Saracco et al.;Citation14 described gradual reduction of this substitutive therapy guided by laboratory assessment recovery of HPA axis and adrenal reserve.
Hyperglycemia may occur as a complication in patients with ALL during induction with steroids and asparginase with reported incidence of 10%.Citation22 In our study, 15 and 12% of patients developed overt hyperglycemia during inductions 1 and 2, respectively. In the study of Sonabend et al.,Citation23 they found that 35% of patients had overt hyperglycemia.
Conclusion
Adrenal suppression is an inevitable consequence of ALL therapy. Monitoring of cortisol levels and steroid coverage during stress is recommended, and gradual steroid tapering is suggested.
Disclaimer statements
Contributors No external contributors.
Funding None.
Conflicts of interest None.
Ethics approval The study was approved by Ain Shams Medical Faculty Ethical Committee.
References
- Gaynon PS, Lustig RH. The use of glucocorticoids in acute lymphoblastic leukemia of childhood. Molecular, cellular and clinical considerations. J Pediatr Hematol Oncol. 1995;17(1):1–12.
- Schimmer BP, Parker KL. Adrenocorticotropic hormone: adrenocortical steroids and their synthetic analogs: Inhibitors of the synthesis and action of adrenocortical hormones. In: Hardman JG, Limbird LE, (eds.) Goodman and Gillman's. The pharmacological basis of therapeutics. 9th ed. New York: Mc Graw Hill 1996. p. 1459–85.
- Schlaghecke R, Kornely E, Santen R. The effect of long term glucocrticoid therapy on pituitary adrenal responses to exogenous corticotrophin-releasing hormone. N Engl J Med. 1992;326:226–30.
- Bangar V, Clayton RN. How reliable is the short synacthen test for the investigation of the hypothalamic pituitary adrenal axis. Eur J Endocrinol. 1998;139(6):580–3.
- Oelkers W. The role of high and low dose corticotropin tests in the diagnosis of secondary adrenal insufficiency. Eur J Endocrinol. 1998;139:567–70.
- Thaler LM, Belvins LS Jr. The low dose (1 ug) adrenocorticotropin stimulation test in the evaluation of patients with suspected central adrenal insufficiency. J Clin Endocrinol Metab. 1998;83(8):2726–9.
- Parker LN. Control of adrenal androgen secretion. Endocrinol Metab Clin North Am. 1991;20(2):401–21.
- Lamberts SW, Bruining HA, de Jong FH. Corticosteroid therapy in severe illness. N Engl J Med. 1997;337:1285–92.
- Naito Y, Fukata J, Tamai S, Seo N, Nakai Y, Mori K, et al. Biphasic changes in hypothalamo-pituitary adrenal function during the early recovery period after major abdominal surgery. J Clin Endocrinol Metab. 1991;73:111–7.
- Graber AL, Ney RL, Nicholson WE, Island DP, Liddle GW. Natural history of pituitary adrenal recovery following long term suppression with corticosteroids. J Clin Endocrinol mETAB. 1965;25:11–6.
- Felner EI, Thompson MT, Ratliff AF, White PC, Dickson BA. Time course of recovery of adrenal function in children treated for leukemia. J Pediatr. 2000;137:21–4.
- Rix M, Birkebaek NH, Rosthoj S, Clausen N. Clinical impact of corticosteroid induced adrenal suppression during treatment for acute lymphoblastic leukemia in children: a prospective observational study using the low dose adrenocorticotropin test. J Pediatr. 2005;147:645–50.
- Margolin L, Cope DK, Bakst-Sisser R, Greenspan J. The steroid withdrawal syndreome: a review of the implications, etiology and treatments. J Pain Symptoms Manage. 2007;33:224–8.
- Saracco P, Bertorello N, Farinasso L, Einaudi S, Barisone E, Altare F, et al. Steroid withdrawal syndrome during steroid tapering in childhood ALL. A controlled study comparing prednisone versus dexamethasone in induction phase. J Pediatr Hematol Oncol. 2005;27(3):141–4.
- Petersen KB, Jusko WJ, Rasmussen M, Schmiegelow K. Population pharmacokinetics of prednisolone in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2003;51:465–73.
- Mahachoklertwattana P, Vilaiyuk S, Hongeng S, Okascharoen C. Suppression of adrenal function in children with acute lymphoblastic leukemia following induction therapy with corticosteroid and other cytotoxic agents. J Pediatr. 2004;144:736–40.
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39:327–48.
- Nasrallah MP, Arafa BM. The value of dehydroepiandrosterone sulfate measurements in the assessment of adrenal function. J Clin Endocrinol Metab. 2003;88:5293–8.
- Bostrom BC, Sensel MR, Sather HN, Gaynon PS, La MK, Johnston K, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's cancer Group. Blood 2003;101:3809–17.
- Shibata S, Kami M, Kanda Y, Machida U, Iwata H, Kishi Y, et al. Acute adrenal failure associated with fluconazole after administration of high dose cyclophosphamide. Am J Hematol. 2001;66(4):303–5.
- Balgaumi AF, Al-Bakrah M, Al-Mahr M, et al. Dexamethasone-associated toxicity during induction chemotherapy for childhood acute lymphoblastic leukemia is augmented by concurrent use of daunomycin. Cancer 2003;97:2898–903.
- Wang YJ, Chu HY, Shu SG, Chi CS. Hyperglycemia induced by chemotherapeutic agents used in acute lymphoblastic leukemia: report of three cases. Zhonghua Yi Xue Za Zhi (Taipei),1993;51(6):457–61.
- Sonabend RY, McKay SV, Okcu MF, Yan J, Haymond MW, Margolin JF. Hyperglycemia during induction therapy is associated with increased infectious complications in childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2008;51(3):387–92.