Abstract
Objectives
Limited data are available on the effect of how cyclophosphamide (CY) and total body irradiation (TBI) are administered. We analyzed the effect of the interval from TBI to hematopoietic stem cell transplantation (HSCT) on the outcome of HSCT.
Methods
Adult patients who underwent HSCT using myeloablative conditioning consisting of TBI and CY were retrospectively analyzed. They were divided into three groups according to the duration between the start of TBI and HSCT (Group A: 2–4 days, Group B: 5–8 days, Group C: 9–10 days).
Results
Seventy-five adult patients were included. The 3-year overall survival rate was 56, 47, and 77% in Groups A, B, and C, respectively (P = 0.14). Similarly, there was no significant difference among the three groups with respect to progression-free survival (57, 47, and 72%, P = 0.17), relapse rate (32, 37, and 16%, P = 0.29), or non-relapse mortality (8, 14, and 12%, P = 0.81). In addition, we observed no significant difference among the three groups with respect to the incidence of grade II–IV acute graft-versus-host disease (GVHD) (31, 47, and 32%, respectively, P = 0.56) and that of chronic GVHD (23, 23, and 22%, respectively, P = 0.97).
Discussion and conclusion
Although recipient immune system at HSCT might be affected by the timing of TBI, the duration between the start of TBI and HSCT did not influence the outcome of HSCT using myeloablative conditioning with TBI and CY.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative treatment strategy for hematopoietic malignancies. The conditioning regimen before HSCT plays an important role in suppressing host immunity to permit donor cell engraftment and the eradication of tumor cells. The combination of high-dose cyclophosphamide (CY) and total body irradiation (TBI) has long been one of the most commonly used myeloablative conditioning regimens.Citation1–Citation6 Initially, high-dose CY was added before TBI to prevent severe tumor lysis syndrome (TLS).Citation7 However, the order and timing of CY and TBI now vary between institutions. These differences might affect the outcome of HSCT in various ways. For example, graft-versus-host disease (GVHD), which is one of the most important complications in HSCT, is considered to be associated with residual host antigen-presenting cells (APCs) which directly present host antigens to donor T cells.Citation8 Since the number of recipient APCs on the day of HSCT may depend on the duration between TBI and HSCT, the timing of TBI might affect the incidence of GVHD.Citation9–Citation10 However, limited data are available on the effect of how CY and TBI are administered.Citation11 In this study, we retrospectively analyzed the effect of the interval from TBI to HSCT on the outcome of HSCT.
Patients and methods
Patients and transplantation procedure
We retrospectively analyzed the records of consecutive adult patients who underwent HSCT at our institution between January 2007 and May 2012 using myeloablative conditioning consisting of TBI (2 Gy twice daily for 3 days) preceded or followed by CY (60 mg/kg for 2 days). Patients who received anti-thymocyte globulin or alemtuzumab in addition to CY and TBI for in vivo T-cell depletion were excluded. GVHD prophylaxis consisted of cyclosporine (CSA) or tacrolimus (TAC) and short-term methotrexate. CSA or TAC was administered as a 24-hour continuous infusion. The dose of CSA and TAC was adjusted to maintain the blood concentration at 500 and 15 ng/ml for standard-risk patients or at 300 and 15 ng/ml for high-risk patients, respectively. Patients were divided into three groups according to the duration between the start of TBI and HSCT. Group A included patients who underwent TBI 2–4 days before HSCT, Group B included those who underwent TBI 5–8 days before HSCT, and Group C included those who underwent TBI 9–10 days before HSCT. CY preceded TBI in Group A, and TBI preceded CY in Groups B and C. The timing of TBI was determined mainly based on the availability of the facility for TBI, rather than on the background of the patients.
TLS was diagnosed according to the Cairo-Bishop definition of laboratory TLS.Citation12–Citation14 In brief, patients were considered to have TLS if 2 or more of these abnormal laboratory findings in blood, i.e., an increased level of uric acid, potassium, or phosphate, or a decreased level of calcium that appeared between 3 days before and 7 days after the start of the conditioning regimen. Toxicities associated with the conditioning regimen were evaluated according to Bearman's grade. Acute GVHD was diagnosed based on clinical criteria with histopathologic confirmation when possible.Citation15 Chronic GVHD was graded by the Seattle criteria based on morphologic manifestations in patients who were alive without relapse for more than 100 days.Citation16 This study was approved by the Institutional Review Board of Saitama Medical Center, Jichi Medical University.
Statistical analysis
Differences in baseline variables among groups were examined using Fisher's exact test for categorical variables and the Kruskal–Wallis test for continuous variables. Overall survival was calculated from the date of HSCT to the date of death from any cause or date of last follow-up using the Kaplan–Meier method, and was compared using the log-rank test. A post hoc multiple comparison test using the Holm method was performed for comparisons among the three groups. Progression-free survival was defined from the date of HSCT to the earliest date of relapse or progression, death from any cause, or last follow-up. The cumulative incidences of relapse and non-relapse mortality were calculated using Gray's method considering each other event as a competing risk, and the cumulative incidences of acute and chronic GVHD were calculated using Gray's method considering progression, relapse, or death before the onset of acute and chronic GVHD, respectively, as competing risks.Citation17 The white blood cell (WBC) count and lymphocyte count at HSCT were analyzed for ordered differences among groups using the Jonckheere-Terpstra test, and were analyzed for relationships with the incidence of GVHD using Fine and Gray's method.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, 2012),Citation18 which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0).
Results
Patient characteristics
A total of 75 patients were included in this study. Groups A, B, and C included 26, 30, and 19 patients, respectively. The characteristics of the patients in each group are summarized in . The median follow-up duration from HSCT for survivors was 1176 days. No significant differences were identified among the three groups.
Table 1. Patient characteristics
Regimen-related toxicity
Although this analysis included 23 patients (31%) who were not in hematological remission at HSCT, laboratory TLS was not observed in any of the 3 groups. With regard to regimen-related toxicities of at least grade 2 as evaluated according to Bearman's grade, mucositis in the oral cavity was most frequently observed in all three groups (89% vs. 93% vs. 95%, respectively). No significant difference was observed in the frequency of these toxicities among the three groups (). Veno-occlusive disease was not observed in any of the three groups, either.
Table 2. Transplantation results
White blood cell count and lymphocyte count
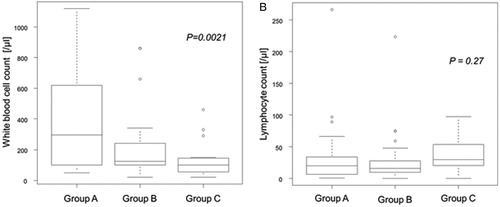
The median WBC count at HSCT was 295, 125, and 100/μl, in Groups A, B, and C, respectively. The WBC count at HSCT showed a statistically significant trend among the three groups based on the duration between TBI and HSCT by the Jonckheere-Terpstra test (P = 0.0021) (A). On the other hand, the median lymphocyte count at HSCT was 20, 16, and 30/μl, in Groups A, B, and C, respectively, and there was no statistically significant trend in the lymphocyte count at HSCT among the three groups (P = 0.27) (B).
Figure 1. White blood cell count and lymphocyte count at transplantation. Box and whisker plots of the white blood cell count at transplantation (A) and the lymphocyte count at transplantation (B) grouped according to the duration between the start of total body irradiation and transplantation (Group A: 2–4 days, Group B: 5–8 days, and Group C: 9–10 days).
Transplantation outcome
All patients except for two patients who died on day 15 (Group A) and day 22 (Group C) after HSCT achieved neutrophil engraftment. The median duration between HSCT and neutrophil engraftment was 21, 22, and 21 days in Groups A, B, and C, respectively (P = 0.56).
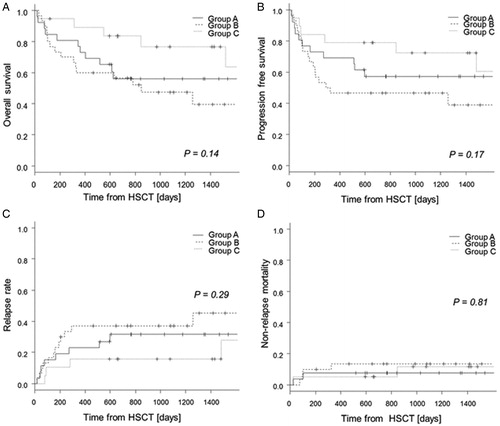
The 3-year overall survival rate was 56% (95% confidence interval (CI): 35–73%), 47% (95% CI: 28–65%), and 77% (95% CI: 49–91%) in Groups A, B, and C, respectively (P = 0.14) (A). In multiple comparisons using the Holm test, there was no significant difference between any pair of groups. Similarly, there was no significant difference among the three groups with respect to progression-free survival (57, 47, and 72%, P = 0.17) (B), relapse rate (32, 37, and 16%, P = 0.29) (C), or non-relapse mortality (8, 14, and 12%, P = 0.81) (D). Multiple comparison tests did not show a significant difference between any pair of groups. On the other hand, the hazard ratios for overall survival adjusted for age, sex, disease status, and donor type were 1.52 (95% CI: 0.68–3.4, P = 0.31) and 0.29 (95% CI: 0.09–0.97, P = 0.04) for Groups B and C, respectively, with Group A as a reference. The hazard ratios for progression-free survival, adjusted for age, sex, disease status, and donor type, were 1.65 (95% CI: 0.73–3.70, P = 0.23) and 0.51 (95% CI: 0.17–1.50, P = 0.22) for Groups B and C, respectively, with Group A as a reference.
Figure 2. Transplantation outcomes. Overall survival curves (A), progression-free survival curves (B), cumulative incidence of relapse (C), and cumulative incidence of non-relapse mortality (D) grouped according to the duration between the start of total body irradiation and transplantation (Group A: 2–4 days, Group B: 5–8 days, and Group C: 9–10 days).
We observed no significant difference among the three groups with respect to the incidence of grade II to IV acute GVHD (31, 47, and 32%, respectively, P = 0.56). In patients who were surviving without relapse or progression at least 100 days after HSCT, there was no difference among the three groups in the incidence of chronic GVHD (23, 23, and 22%, respectively, P = 0.97). No significant relationships were observed between the incidence of grade II to IV acute GVHD and WBC or lymphocyte count at HSCT (P = 0.63, 0.80, respectively). There were also no significant differences between chronic GVHD and either WBC or the lymphocyte count at HSCT (P = 0.44, 0.40, respectively). To analyze the impact of the order of TBI and CY, Groups B and C were combined into Group BC, in which TBI preceded CY. There were no significant differences in major transplantation outcomes, including overall survival, progression-free survival, and the incidences of acute and chronic GVHD even if the hazard ratios for these outcomes were adjusted for age, sex, disease status, and donor type (data not shown).
Discussion
In this study, we retrospectively evaluated the effect of the order of CY and TBI and the duration between the start of TBI and HSCT on the outcome of HSCT. With regard to the combination of CY and busulfan, the order of these two agents has been reported to be associated with regimen-related toxicity. The administration of CY prior to busulfan reduced liver toxicity, compared with the opposite order for the administration of these drugs, in mouse and human HSCT.Citation19–Citation21 In these studies, the authors supposed that the reduced liver toxicity might be attributed to the interaction of these two drugs, which affected the pharmacokinetics of CY. On the other hand, the timing of TBI is not considered to affect the pharmacokinetics of CY, and in fact, no significant difference was observed in the frequency of any toxicity among the different timings of TBI in this study. In addition, TLS was not observed, regardless of the timing and order of TBI, even though about 30% of patients in each group underwent HSCT in non-remission. This result was not compatible with the old prediction of Fefer et al.Citation7 They administered CY before TBI to avoid TLS. The lack of differences regarding neutrophil engraftment might suggest that the duration between TBI and HSCT did not influence the suppression of host immunity that may disturb donor cell engraftment.
Residual recipient APCs are believed to be associated with the incidence of GVHD.Citation8 Although some molecular markers of APCs have been reported, these markers have not been used in clinical settings.Citation22–Citation24 In this study, we used the WBC and lymphocyte counts at HSCT as surrogate values to estimate the amount of residual recipient APCs. The duration between the start of TBI and HSCT was associated with the WBC count at HSCT, but not with the lymphocyte count. This difference is probably attributable to the fact that the lymphocyte count decreases immediately after TBI, before the suppression of other subsets of WBC.Citation25 On the other hand, the incidence of grade II to IV acute GVHD and chronic GVHD was not significantly different among the three groups, and, in addition, the WBC count and lymphocyte count at HSCT were not associated with the incidence of GVHD. These results might demonstrate that both the WBC and lymphocyte counts at HSCT did not reflect the amount of residual recipient APCs. Alternatively, it has recently been shown that nonhematopoietic APCs were associated with the occurrence of GVHD, irrespective of the profound depletion of recipient hematopoietic APCs.Citation26–Citation27
Kato et al.Citation11 reported that TBI performed before chemotherapy in the conditioning regimen before HSCT was associated with an increased risk of grade II to IV acute GVHD, compared with TBI performed after chemotherapy, in pediatric patients. They stated that TBI performed before chemotherapy might induce more severe tissue damage and elevate inflammatory cytokine secretion, which could lead to an increased incidence of grade II to IV acute GVHD. However, in our study, the order of CY and TBI and the timing of TBI did not affect the incidence of grade II to IV acute GVHD. This discrepancy may be partly due to the difference between pediatric HSCT and adult HSCT. Another explanation is that we used GVHD prophylaxis with a higher target blood concentration of CSA, which might mask the effect of the conditioning regimen.Citation28 The lack of a significant effect of the order of TBI and CY on overall survival, progression-free survival, relapse rate, and non-relapse mortality in their pediatric study was consistent with our findings.
Overall survival in Group C was significantly superior to that in Group A, after adjusting for age, sex, disease status, and donor type by a multivariate analysis. Since there were no differences in relapse rate, non-relapse mortality, incidence of acute GVHD, or incidence of chronic GVHD among the three groups even after adjusting for other important factors, we could not clarify the reason for the superior overall survival in Group C. This result will require validation in another cohort.
In conclusion, the duration between the start of TBI and HSCT and the order of TBI and CY did not influence the outcome of HSCT using myeloablative conditioning with TBI and CY. Further analyses, including the serial evaluation of biomarkers, are warranted to assess the impact of the duration between the start of TBI and HSCT.
Disclaimer statements
Contributors YA drafted the paper. All authors contributed to revising the paper critically for important intellectual content, and approved the final version to be submitted. YA, SK and YK designed the study and analyzed data. All authors participated in data collection.
Funding None.
Conflicts of interest None.
Ethics approval This study was approved by the Institutional Review Board of Saitama Medical Center, Jichi Medical University.
References
- Ringden O, Labopin M, Tura S, Arcese W, Iriondo A, Zittoun R, et al. A comparison of busulphan versus total body irradiation combined with cyclophosphamide as conditioning for autograft or allograft bone marrow transplantation in patients with acute leukaemia. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 1996;93(3):637–45.
- Kroger N, Zabelina T, Kruger W, Renges H, Stute N, Kabisch H, et al. Comparison of total body irradiation vs busulfan in combination with cyclophosphamide as conditioning for unrelated stem cell transplantation in CML patients. Bone Marrow Transplant. 2001;27(4):349–54.
- Shi-Xia X, Xian-Hua T, Hai-Qin X, Bo F, Xiang-Feng T. Total body irradiation plus cyclophosphamide versus busulphan with cyclophosphamide as conditioning regimen for patients with leukemia undergoing allogeneic stem cell transplantation: a meta-analysis. Leuk Lymphoma 2010;51(1):50–60.
- Weiden PL, Storb R, Deeg HJ, Graham TC, Thomas ED. Prolonged disease-free survival in dogs with lymphoma after total-body irradiation and autologous marrow transplantation consolidation of combination-chemotherapy-induced remissions. Blood 1979;54(5):1039–49.
- Clift RA, Radich J, Appelbaum FR, Martin P, Flowers ME, Deeg HJ, et al. Long-term follow-up of a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide for patients receiving allogenic marrow transplants during chronic phase of chronic myeloid leukemia. Blood 1999;94(11):3960–2.
- Kanda Y, Sakamaki H, Sao H, Okamoto S, Kodera Y, Tanosaki R, et al. Effect of conditioning regimen on the outcome of bone marrow transplantation from an unrelated donor. Biol Blood Marrow Transplant. 2005;11(11):881–9.
- Fefer A, Einstein AB, Thomas ED, Buckner CD, Clift RA, Glucksberg H, et al. Bone-marrow transplantation for hematologic neoplasia in 16 patients with identical twins. N Engl J Med. 1974;290(25):1389–93.
- Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 1999;285(5426):412–5.
- Banovic T, Markey KA, Kuns RD, Olver SD, Raffelt NC, Don AL, et al. Graft-versus-host disease prevents the maturation of plasmacytoid dendritic cells. J Immunol. 2009;182(2):912–20.
- Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke KR, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8(6):575–81.
- Kato M, Shiozawa R, Koh K, Nagatoshi Y, Takita J, Ida K, et al. The effect of the order of total body irradiation and chemotherapy on graft-versus-host disease. J Pediatr Hematol Oncol. 2014; 36(1): e9–12.
- Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin's lymphoma. Am J Med. 1993;94(2):133–9.
- Del Toro G, Morris E, Cairo MS. Tumor lysis syndrome: pathophysiology, definition, and alternative treatment approaches. Clin Adv Hematol Oncol. 2005;3(1):54–61.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127(1):3–11.
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974;18(4):295–304.
- Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–17.
- Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706.
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
- Nilsson C, Forsman J, Hassan Z, Abedi-Valugerdi M, O'Connor C, Concha H, et al. Effect of altering administration order of busulphan and cyclophosphamide on the myeloablative and immunosuppressive properties of the conditioning regimen in mice. Exp Hematol. 2005;33(3):380–7.
- Sadeghi B, Jansson M, Hassan Z, Mints M, Hagglund H, Abedi-Valugerdi M, et al. The effect of administration order of BU and CY on engraftment and toxicity in HSCT mouse model. Bone Marrow Transplant. 2008;41(10):895–904.
- Kerbauy FR, Tirapelli B, Akabane H, Oliveira JS. The effect of administration order of BU and CY on toxicity in hematopoietic SCT in humans. Bone Marrow Transplant. 2009;43(11):883–5.
- Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176(1):47–58.
- Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185(6):1101–11.
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165(11):6037–46.
- Goans RE, Holloway EC, Berger ME, Ricks RC. Early dose assessment in criticality accidents. Health Phys. 2001;81(4):446–9.
- Li H, Demetris AJ, McNiff J, Matte-Martone C, Tan HS, Rothstein DM, et al. Profound depletion of host conventional dendritic cells, plasmacytoid dendritic cells, and B cells does not prevent graft-versus-host disease induction. J Immunol. 2012;188(8):3804–11.
- Koyama M, Kuns RD, Olver SD, Raffelt NC, Wilson YA, Don AL, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat Med. 2012;18(1):135–42.
- Machishima T, Kako S, Wada H, Yamasaki R, Ishihara Y, Kawamura K, et al. The safety and efficacy of acute graft-versus-host disease prophylaxis with a higher target blood concentration of cyclosporine around 500 ng/ml. Clin Transplant. 2013;27(5):749–56.
