Abstract
Objectives
The aim of this study was to assess bone marrow (BM) fibrosis and dysplasia in chronic myeloid leukemia (CML) patients receiving the first-generation tyrosine kinase inhibitor (TKI), imatinib, or second-generation TKIs, dasatinib, and nilotinib. We further investigated whether CML under TKI is associated with dysplastic BM changes during the clinicopathological course of the disease.
Methods
In total, pre-treatment BM paraffin blocks of biopsy specimens were available for 41 adult patients diagnosed with chronic phase CML. Post-treatment BM aspirate clot and core biopsy samples were reviewed for fibrosis and dyshematopoiesis.
Results
Overall, 13 (31.7%) patients achieved a complete cytogenetic response with imatinib treatment, with no events. In 25 patients, imatinib was discontinued owing to primary or secondary resistance. In patients with initial dysmyelopoiesis, the rate of BM fibrosis was 82.4 versus 47.6% for other patient groups (P = 0.02). Overall, 24 patients with newly diagnosed CML showed marrow fibrosis, among which 19 (79.1%) had imatinib resistance. However, only 5 out of 15 patients (33.5%) without marrow fibrosis had imatinib resistance (P = 0.08).
Discussion
Our findings indicate that BM fibrosis is an independent predictor of the ‘TKI drug response level’ in CML and support its inclusion as a critical pathobiological parameter for decision-making with regard to TKI drug selection de novo, calculation of prognosis at the onset of disease, and monitoring response to TKI in the long-term disease course of CML.
Keywords:
Introduction
European LeukemiaNet (ELN) recommendations have been established with regard to the tyrosine kinase inhibitors (TKIs) that should be used as first- and second-line therapy, critical end-points of TKI treatment, and optimal approaches to evaluate disease prognosis and monitor the TKI response in chronic myeloid leukemia (CML).Citation1 Bone marrow (BM) fibrosis is not a currently accepted parameter for critical decision-making regarding TKI drug selection, disease prognosis, end-point selection, and monitoring elements.Citation1 Clinical CML disease presentations are heterogeneous, and therapy is decided on an individual basis.Citation2 The selection of first- versus second-generation TKIs for BCR-ABL1 t(9;22)(q34;q11) suppression, early versus late switch to more powerful TKIs, prediction of patients with a poor prognosis, and early detection of progressing patients are critical factors in the clinical management of CML.Citation1,Citation2 Fibrosis-inducing molecules are important in the pathobiology of CML.Citation3,Citation4 Therefore, the status of BM fibrosis in the expanding clinical spectrum of CML should be elucidated to optimize clinical decision-making and follow-up.
Preliminary data have shown that CML disease may be complicated by the subsequent development of dysplasia.Citation5–Citation8 Thus, BM histopathology should be assessed for dysplastic changes and fibrosis at the onset and during critical time-points in CML patients receiving TKIs. The aim of the current study was to assess BM fibrosis and dysplasia in CML patients receiving first-generation TKI (imatinib) or second-generation TKI (dasatinib or nilotinib). The utility of BM fibrosis as an independent prognostic factor for CML disease risk and in predicting the TKI response level was examined. We further investigated whether CML under TKI is associated with dysplastic BM changes during the clinicopathological course of the disease.
Materials and methods
This study included 41 adult patients diagnosed with chronic phase CML. Adults diagnosed as Ph-positive chronic phase (CP) (CML Department of Hematology, Hacettepe University Medical School) were analyzed. We collected information on demographic features, peripheral blood, and BM analysis at the onset using the Sokal score. Pre-treatment paraffin blocks of BM biopsy specimens were available for these patients (). Cytogenetic responses (CgR) were categorized as minor CgR (Ph 36–95%) and major CgR (Ph 1–35%). BM aspirate clot and core biopsy samples stained with hematoxylin and eosin were reviewed. Samples were subjected to reticulin staining (ammoniacal silver procedure) and graded on a scale of 1–3. Treatment with imatinib was initiated within a few weeks of diagnostic tests on BM aspirate (including morphologic analysis, cytogenetics, and fluorescence in situ hybridization in case of lack of metaphases) to minimize the change in disease status before treatment initiation. In cases where patients in the chronic phase treated with imatinib developed resistance or intolerance to the drug, second-generation TKIs (dasatinib or nilotinib) were used. Post-treatment BM aspirate clot and core biopsy samples were reviewed for fibrosis and dysplasia ().
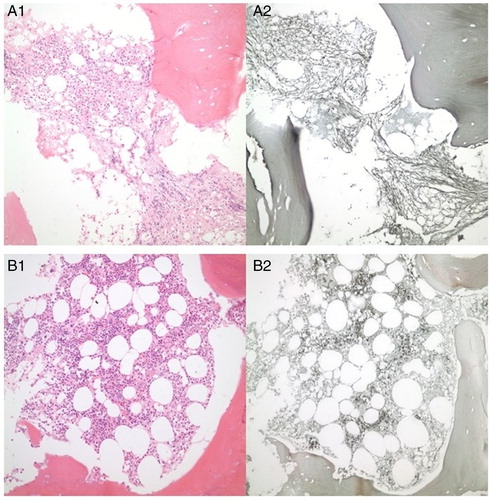
Figure 1. Bone marrow (BM) fibrosis and dysplasia of the de novo CML patient before TKI treatment (A1 and A2). Fibrosis and dysplasia of bone BM have been healed due to the pharmacobiological effects of TKIs after 3 years of imatinib and 1 year of dasatinib treatment (B1 and B2).
Statistical analysis
Variables were compared using the Chi-square test. Significance was set at P < 0.05. Time to failure was measured from the start of therapy until the patients were removed from the study because of death, transformation to accelerated or blastic phases, and cytogenetic or hematologic resistance or toxicity intolerance.
Results
The median age of patients was 49 years (range, 25–73 years), and 22 (53.7%) were female. Reticulin fibrosis before therapy was Grade 1 in 11 patients (26.8%), Grade 2 in 11 patients (26.8%), and Grade 3 in 2 patients (4.9%). Data are summarized in . In total, 13 (31.7%) patients achieved complete CgR with imatinib treatment, with no events. Twenty-eight patients (68.3%) discontinued treatment with imatinib mesylate. Treatment was interrupted in three patients owing to toxicity (skin toxicity in one patient and gastrointestinal toxicity in two patients), and in the remaining 25 patients, imatinib was discontinued due to primary or secondary resistance. The second-generation TKIs used for this group were dasatinib for 16 (39%) and nilotinib for 12 patients (29.3%). Data are summarized in .
Table 1. Baseline characteristics of chronic myeloid leukemia patients
Table 2. TKI drug disposition in patients with chronic myeloid leukemia
Severe fibrosis was not associated with the worst risk groups according to the index of Sokal (P = 0.33). CgR rates were similar in patients with absent-mild and severe BM fibrosis. In patients with BM fibrosis, the incidence of complete CgR was 61.5 versus 59% for other groups (P = 0.89), and that of the molecular response was 66.6 versus 81.2% for other groups (P = 0.68). Data are summarized in . We observed an association between marrow fibrosis and poor response to imatinib. Rates of events (cytogenetic or hematologic resistance or treatment resistance toxicity intolerance) were higher in patients with BM fibrosis. Marrow fibrosis in newly diagnosed CML was associated with therapy resistance, but its development on therapy was not related to treatment failure. Furthermore, no association was evident between response to treatment (complete cytogenetic response; CCyR) and dysplasia (dysmyelopoiesis, dysmegakaryopoiesis, and dyserythropoiesis). In patients with initial dysmyelopoiesis, the rate of BM fibrosis was 82.4 versus 47.6% for other groups (P = 0.02). We observed no association between the degree of fibrosis and thrombocytosis, anemia (Hb < 10 g/dL) or dyserythropoiesis. No gender effect on dysplasia was evident. Moreover, there were no differences between TKI therapy effects on resolution of dysplasia. Twenty of 24 patients had imatinib resistance, while only four patients showed dyserythropoiesis. Twenty-four patients with newly diagnosed CML had marrow fibrosis, among which 19 (79.1%) were imatinib-resistant. In contrast, only 5 of 15 patients (33.5%) without marrow fibrosis displayed imatinib resistance (P = 0.08). Following imatinib therapy, we noted resolution of marrow fibrosis in 5 patients (50%), and significant resolution of marrow fibrosis in 14 patients (70%) subjected to second-generation TKI treatment. At the time of the last follow-up, 13 patients had been receiving first-generation TKI (imatinib) therapy for a median of 46.9 months (range, 11–134 months) and 28 patients had been receiving second-generation TKI for a median of 37.7 months (range, 7–87 months).
Table 3. Molecular response rates based on BCR-ABL monitoring via polymerase chain reaction (PCR) in chronic myeloid leukemia patients with and without bone marrow fibrosis
Discussion
In this study, we observed an association between BM fibrosis and poor response to first-generation TKI. The proportion of complicated end-points (cytogenetic or hematologic resistance or treatment resistance toxicity intolerance) were significantly associated with the presence of BM fibrosis. Similarly, marrow fibrosis in newly diagnosed CML was associated with therapy resistance, although its development during therapy was not linked to treatment failure. Fibrosis-inducing molecules are important in the pathobiology of CML.Citation3,Citation4,Citation9 However, BM fibrosis is not an accepted prognostic parameter in all current CML disease prognostic systems, including Sokal, Euro/Hasford, and European Treatment and Outcome Study (EUTOS),Citation10 or for critical decision-making in ELN–CML recommendations for the TKI management of CML.Citation1 Our results on marrow fibrosis of CML indicate that BM histopathology, particularly fibrosis, should be included as a parameter in addition to the established pathological features (spleen size, basophils, age, blast count, BCR-ABL load, and additional cytogenetic abnormalities) in CML. Morphologic criteria for CCyR in patients with CML treated with imatinib have been suggested.Citation11 Persistent high-level BM morphologic abnormalities herald early on a high likelihood of treatment failure and highlight the need for more intensive or alternative therapy.Citation11 However, this morphological approach is not widely accepted at present. The significant resolution of BM fibrosis in 70% of our CML patients on second-generation TKI treatment indicates that more powerful TKI inhibition may effectively improve the marrow microenvironment.
Appropriate selection of first-generation TKI (imatinib) versus second-generation TKI (dasatinib or nilotinib) is an unresolved issue in newly diagnosed CML patients. In view of our present results, de novo CML patients with BM fibrosis appear to be ideal candidates for the initiation of more powerful TKIs (nilotinib or dasatinib) at the beginning after the prediction of a poor prognosis. Similarly, the debate between researchers advocating early (after 3 or 6 months of imatinib) versus late (after 1 year) switch to more powerful TKIsCitation1,Citation2 could be resolved with the detection of BM fibrosis. We propose that marrow fibrosis, regularly assessed during the follow-up of CML patients, is an effective indicator of disease progression.
Conflicting results have been obtained from previous investigations focusing on BM fibrosis in CML.Citation12–Citation22 Early studies in the interferon (IFN) era for CML reported that the effectiveness of IFN-alpha on BM fibrosis depends on treatment intensity. Data from one of these studiesCitation13 showed a reversal of BM fibrosis when combining high-dose IFN-alpha with low-dose Ara-C, but progression upon application of low-dose IFN-alpha. Thus, BM fibrosis may be a significant early indicator of ineffective therapy in CML patients receiving IFN.Citation13 Imatinib is proposed to reverse initial BM fibrosis in CML, but neither eliminates unfavorable prognosis nor completely guarantees against the evolution of BM fibrosis.Citation14 Our results were in agreement with these conclusions. In contrast, Kantarjian et al.Citation21 suggested that previously established poor prognostic significance of marrow fibrosis in CML is less relevant with imatinib therapy. In their study, CML patients with severe BM fibrosis had similar CCyR rates with imatinib treatment (67 versus 58%; P = 0.45), compared to those with mild-to-moderate fibrosis.Citation21 In the study, we observed no association between response to treatment (CCyR) and dysplasia (dysmyelopoiesis, dysmegakaryopoiesis, and dyserythropoiesis). Accordingly, we propose that TKI drugs, even the more powerful BCR-ABL1 inhibitors (dasatinib or nilotinib), do not cause dysplastic BM changes in CML. In view of this positive safety signal for second-generation TKIs, targeted drugs may be an ideal candidate for the management of high-risk CML involving the presence of BM fibrosis.
The biological backgrounds of BM fibrosis in CML have been documented in the literature.Citation3,Citation11,Citation18,Citation23–Citation29 Data on mast cell quantities in different myeloproliferative neoplasms, including CML, suggest a direct role for mast cells in intramedullary fibrosis.Citation23 Pharmacological targeting of c-Abl and its downstream effector pathways may represent a novel therapeutic approach to blocking transforming growth factor (TGF)-beta-dependent fibrotic processes.Citation24 Microvascular density, in association with angiogenesis, tends to increase in CML.Citation25 Basic fibroblast growth factor (bFGF) may represent an important link between angiogenesis, fibrosis, and clonal neoplastic hematopoiesis during the development of myeloproliferative neoplasms, including CML.Citation3 Human fibroblasts are derived from hematopoietic stem cells. Therefore, BM fibrosis in patients with CML may be part of the clonal process.Citation27 Earlier biological perspectives, as well as data from our present study, cast doubt on the inclusion of BM fibrosis as one of the prognostic criteria of CML. Based on our present finding that BM fibrosis is an independent predictor of the ‘TKI drug response level’ in CML, we advocate its inclusion as a pathobiological parameter for critical decision-making regarding TKI drug selection de novo, calculation of prognosis at disease onset, and monitoring the response to TKI in the long-term disease course of CML.
Disclaimer statements
Contributors All authors contributed equally to this work.
Funding None.
Conflicts of interest None.
Ethics approval The ethical approval was obtained from Hacettepe University, Ankara, Turkey.
References
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013;122:872–84.
- Haznedaroglu IC. Current concerns of undertreatment and overtreatment in chronic myeloid leukemia based on European LeukemiaNet 2013 recommendations. Expert Opin Pharmacother. 2013;14:2005–10.
- Sayinalp N, Cinar H, Uner A, Haznedaroglu IC, Buyukasik Y, Goker H, et al. Plasma basic fibroblast growth factor and bone marrow fibrosis in clonal myeloproliferative disorders. Clin Lab Haematol. 2004;26:265–8.
- Cobankara V, Oran B, Ozatli D, Haznedaroglu IC, Kosar A, Buyukasik Y, et al. Cytokines, endothelium, and adhesive molecules in pathologic thrombopoiesis. Clin Appl Thromb Hemost. 2001;7:126–30.
- Bennour A, Tabka I, Ben Youssef Y, Kmeria Z, Khelif A, Saad A, et al. A novel t(3;12)(q21;p13) translocation in a patient with accelerated chronic myeloid leukemia after imatinib and nilotinib therapy. Cancer Biol Med. 2013;10:47–51.
- Karimata K, Masuko M, Ushiki T, Kozaki T, Shibasaki Y, Yano T, et al. Myelodysplastic syndrome with Ph negative monosomy 7 chromosome following transient bone marrow dysplasia during imatinib treatment for chronic myeloid leukemia. Intern Med. 2011;50:481–5.
- Navarro JT, Feliu E, Grau J, Espiret B, Colomer D, Ribera JM, et al. Monosomy 7 with severe myelodysplasia developing during imatinib treatment of Philadelphia-positive chronic myeloid leukemia: two cases with a different outcome. Am J Hematol. 2007;82:849–51.
- Pitini V, Arrigo C, Sauta MG, Altavilla G. Myelodysplastic syndrome appearing during imatinib mesylate therapy in a patient with GIST. Leuk Res. 2009;33:e143–4.
- Uz B, Tatonyan SC, Sayitoglu M, Erbilgin Y, Ng OH, Buyukasik Y, et al. Local hematopoietic renin-angiotensin system in myeloid versus lymphoid hematological neoplastic disorders. J Renin Angiotensin Aldosterone Syst. 2013;14:308–14.
- Uz B, Buyukasik Y, Atay H, Kelkitli E, Turgut M, Bektas O, et al. EUTOS CML prognostic scoring system predicts ELN-based ‘event-free survival’ better than Euro/Hasford and Sokal systems in CML patients receiving front-line imatinib mesylate. Hematology 2013;18:247–52.
- Lugli A, Ebnoether M, Cogliatti SB, Gratwohl A, Passweg J, Hess U, et al. Proposal of a morphologic bone marrow response score for imatinib mesylate treatment in chronic myelogenous leukemia. Hum Pathol. 2005;36:91–100.
- Beham-Schmid C, Apfelbeck U, Sill H, Tsybrovsky O, Höfler G, Haas OA, et al. Treatment of chronic myelogenous leukemia with the tyrosine kinase inhibitor STI571 results in marked regression of bone marrow fibrosis. Blood 2002;99:381–3.
- Buesche G, Freund M, Hehlmann R, Georgii A, Ganser A, Hecker H, et al. Treatment intensity significantly influencing fibrosis in bone marrow independently of the cytogenetic response: meta-analysis of the long-term results from two prospective controlled trials on chronic myeloid leukemia. Leukemia 2004;18:1460–67.
- Buesche G, Ganser A, Schlegelberger B, von Neuhoff N, Gadzicki D, Hecker H, et al. Marrow fibrosis and its relevance during imatinib treatment of chronic myeloid leukemia. Leukemia 2007;21:2420–27.
- Buesche G, Georgii A, Duensing A, Schmeil A, Schlue J, Kreipe HH. Evaluating the volume ratio of bone marrow affected by fibrosis: a parameter crucial for the prognostic significance of marrow fibrosis in chronic myeloid leukemia. Hum Pathol. 2003;34:391–401.
- Buesche G, Hehlmann R, Hecker H, Heimpel H, Heinze B, Schmeil A, et al. Marrow fibrosis, indicator of therapy failure in chronic myeloid leukemia – prospective long-term results from a randomized-controlled trial. Leukemia 2003;17:2444–53.
- Bueso-Ramos CE, Cortes J, Talpaz M, O'Brien S, Giles F, Rios MB, et al. Imatinib mesylate therapy reduces bone marrow fibrosis in patients with chronic myelogenous leukemia. Cancer 2004;101:332–6.
- de Jesus CR, I-Ching L, Carvalho Neiva TdJ, Vituri CdL. Assessment of fibrosis and vascularization of bone marrow stroma of chronic myeloid leukemia patients treated with imatinib mesylate and their relationship with the cytogenetic response. Braz J Pharm Sci. 2011;47:313–22.
- Frater JL, Tallman MS, Variakojis D, Druker BJ, Resta D, Riley MB, et al. Chronic myeloid leukemia following therapy with imatinib mesylate (Gleevec). Bone marrow histopathology and correlation with genetic status. Am J Clin Pathol. 2003;119:833–41.
- Kantarjian HM, Bueso-Ramos CE, Talpaz M, O'Brien S, Giles F, Faderi S, et al. Significance of myelofibrosis in early chronic-phase, chronic myelogenous leukemia on imatinib mesylate therapy. Cancer 2005;104:777–80.
- Kantarjian HM, Bueso-Ramos CE, Talpaz M, O'Brien S, Giles F, Rios MB, et al. The degree of bone marrow fibrosis in chronic myelogenous leukemia is not a prognostic factor with imatinib mesylate therapy. Leuk Lymphoma 2005;46:993–7.
- Khonglah Y, Basu D, Dutta TK. Bone marrow trephine biopsy findings in chronic myeloid leukemia. Malays J Pathol. 2002;24:37–43.
- Ahmed A, Powers MP, Youker KA, Rice L, Ewton A, Dunphy CH, et al. Mast cell burden and reticulin fibrosis in the myeloproliferative neoplasms: a computer-assisted image analysis study. Pathol Res Pract. 2009;205:634–8.
- Bhattacharyya S, Ishida W, Wu M, Wilkes M, Mori Y, Hinchcliff M, et al. A non-Smad mechanism of fibroblast activation by transforming growth factor-beta via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene 2009;28:1285–97.
- Mahadevan KK, Basu D, Kumar S. Does CD34 staining reflect the angiogenic process in the bone marrow? An analysis of a series of chronic myeloid Leukemia patients. J Clin Diagn Res. 2014;8:FC04–7.
- Rao S, Sen R, Singh S, Ghalaut PS, Arora BB. Grading of marrow fibrosis in chronic myeloid leukemia – a comprehensive approach. Indian J Pathol Microbiol. 2005;48:341–4.
- Shirai K, Sera Y, Bulkeley W, Mehrotra M, Moussa O, LaRue AC, et al. Hematopoietic stem cell origin of human fibroblasts: cell culture studies of female recipients of gender-mismatched stem cell transplantation and patients with chronic myelogenous leukemia. Exp Hematol. 2009;37:1464–71.
- Srinivas BH, Paul TR, Uppin SG, Uppin MS, Jacob RT, Raghunadharao D. Morphologic changes in the bone marrow in patients of chronic myeloid leukemia (CML) treated with imatinibmesylate. Indian J Hematol Blood Transfus. 2012;28:162–9.
- Valent P, Agis H, Sperr W, Sillaber C, Horny HP. Diagnostic and prognostic value of new biochemical and immunohistochemical parameters in chronic myeloid leukemia. Leuk lymphoma 2008;49:635–8.
