Abstract
Objective
To determine the efficacy of hydroxyurea therapy on transfusion-dependent beta-thalassemia patients.
Methods
This study collected articles from databases, applied inclusion and exclusion criteria, and analyzed them for bias. The effects of hydroxyurea on transfusion requirements were categorized according to the following definitions. ‘Good responders’ were participants who became transfusion independent after treatment. ‘Moderate responders’ were participants who were still transfusion dependent, however, experienced a significant decline in their transfusion requirements. ‘Poor responders’ were defined as participants who did not respond to hydroxyurea.
Results
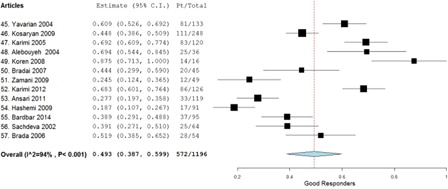
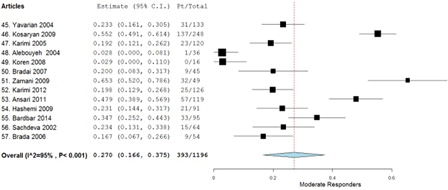
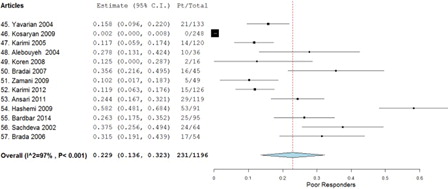
A total of 13 studies met all the inclusion and exclusion criteria providing a total of 1196 study participants. The weighted average of the odds ratio using the random effect model (P < 0.001) was determined to be 0.493 for good responders, 0.270 for moderate responders, and 0.229 for poor responders.
Discussion
Until now, there has not been any double-blinded placebo-controlled studies performed looking at the effectiveness of hydroxyurea with this regard, and this substantially limits this meta-analysis. More studies should be conducted to determine whether hydroxyurea preferentially treats particular type of mutations over others.
Conclusion
This study suggests that hydroxyurea provides some benefit to patients, and therefore, in certain clinical situations, it may be understandable to start a trial therapy of hydroxyurea to qualifying patients. However, a double-blinded placebo-controlled studies should be performed before its efficacy can be considered established.
Introduction
Beta-thalassemia syndromes are characterized by a genetic defect in the synthesis of beta-globin chains of the hemoglobin protein. This hereditary disorder, in the homozygous state, causes beta-thalassemia major, a severe hemolytic disorder that renders the patient blood transfusion dependent. The heterozygous state, also known as beta-thalassemia minor, may produce symptoms ranging from mild to moderate anemia. These patients may require blood transfusions as well, depending upon the severity of their anemia. Despite iron chelation therapy, iron-overload greatly reduces the life expectancy and quality of life of the patients.
The use of hydroxyurea to induce the production of fetal hemoglobin in patients with sickle cell anemia has been successful in relieving their symptoms. Using this principle, studies have been conducted to determine whether the use of hydroxyurea in patients with beta-thalassemia will provide similar benefit. Some studies have shown significant reduction in the need for blood transfusions and iron chelators, while others have shown little effect. Therefore, currently there is no conclusive study validating or falsifying whether hydroxyurea can reduce or abolish the transfusion and chelation requirements in patients with beta-thalassemia. Thus, the inclusion of hydroxyurea in the management of thalassemia remains in doubt. An aggregated analysis of the data available may allow us to draw definitive conclusion on the efficacy of hydroxyurea to reduce transfusion requirements in patients with transfusion-dependent beta-thalassemia. If it is found to be effective in reducing the frequency of transfusions, it could substantially reduce the transfusion-related morbidity and mortality, and significantly improve the quality of life of the patients suffering from thalassemia.
Methodology
Study design
Through a systematic methodology, this study collected, identified, and assessed online articles and applied various statistical approaches to combine the results from the multiple studies in an effort to improve the precision and accuracy of individual findings and/or resolve conflicts or uncertainties between contradicting studies. The study design incorporated the methods and guidelines proposed by MOOSECitation1 and PRISMACitation2 in the execution of this meta-analysis.
The articles analyzed in this meta-analysis are interventional clinical trials administering hydroxyurea to transfusion-dependent patients diagnosed with beta-thalassemia. In these studies, the patient's transfusion requirements prior to the application of hydroxyurea therapy serve as the control and the change in transfusion requirement after hydroxyurea therapy is the dependent variable. Unless the study specifically mentioned a change in transfusion protocol between pre-treatment and post-treatment, this meta-analysis assumed that the protocols were consistent throughout.
Article collection
The guidelines established in the PRISMA 2009 flow diagram presented by the PRISMA GroupCitation2 were applied during the article collection process.
Identification
An initial sample of articles was derived by using the search words ‘thalassemia hydroxyurea’ in the search engine ‘Google Scholar’ (http://scholar.google.com). As of June 2014, exactly 6250 peer-reviewed articles were generated from various journals, reports, books, and other documents. Identical searches through MEDLINE and Cochrane did not produce any additional results. Our initial sample was confined completely to Internet searches, and it was not necessary to contact the authors directly.
Screening
Titles of approximately 650 articles from the initial sample of 6250 articles were screened, and any articles containing the words ‘thalassemia’ and ‘hydroxyurea’ in the title were selected for further investigation.
Eligibility
Inclusion
The following inclusion criteria were applied. Articles that did not meet the following inclusion criteria were removed from qualitative and quantitative analysis:
1. Human trials – only human-based trials were accepted;
2. Clinically based – all studies must have been performed in a clinical setting;
3. Interventional studies – only studies providing patients with hydroxyurea and examining its effects prospectively were used in this study.
Exclusion
Any article that met the following exclusion criteria was excluded from quantitative analysis:
| 1. | Missing information: articles missing any of the following information were excluded from this study:
| ||||
| 2. | Transfusion independent: studies done solely with transfusion independent subjects were excluded. Any studies that contained both dependent and independent subjects, but were placed in separate groups, were still included; however, only the information relevant to the transfusion-dependent group was extracted; | ||||
| 3. | Other interventions: articles that combined the use of hydroxyurea with another therapy (EPO, phenylbutyrate, etc.) were also be excluded; | ||||
| 4. | Coexisting diseases: articles studying patients with other blood or bone marrow diseases (sickle cell, etc.) were excluded from the analysis. When the author did not mention other medical conditions, it was assumed that the patients did not have co-existing conditions; | ||||
| 5. | Less than five patients: articles that consisted of less than five patients, including case studies, were excluded; | ||||
| 6. | Drug dosage: studies with hydroxyurea dosages ranging outside 10–20 mg/kg/day were excluded; | ||||
| 7. | Languages other than English: articles that were in languages other than English were excluded from this study; | ||||
| 8. | Bias: bias was assessed at the study level. Articles that did not receive 4 out of 4 stars from the Newcastle–Ottawa Quality Assessment Scale for Case Control StudiesCitation3 were excluded from the study. | ||||
Analytic approach
Efficacy of hydroxyurea therapy is measured by the degree of decreased blood transfusion requirements of patients. Hemoglobin values were not used as a measurement of treatment efficacy, because pre-treatment blood transfusions artificially elevate the pre-treatment hemoglobin levels. Since pre-treatment hemoglobin values can not serve as a historical control, they were not included in the statistical analysis.
The effects of hydroxyurea on transfusion requirements were categorized according to the following definitions:
| 1. | Good responders – patients became transfusion independent or experienced a decrease in transfusion rate greater than 70% after treatment with hydroxyurea; | ||||
| 2. | Moderate responders – patients were still transfusion dependent; however, there was a significant decline in their transfusion requirements after hydroxyurea administration; | ||||
| 3. | Poor responders – patients did not respond to hydroxyurea treatment or experienced a very minimal response to treatment (less than 40%). | ||||
Patients from all included studies were distributed into the applicable group, and subsequent statistical modeling was performed.
Statistical approach
An initial summary statistics were calculated using the observation ratio of each of the three groups: good responders, moderate responders, and poor responders. Subsequently, the overall treatment effect ratio as a weighted average of the summary statistics was calculated. The weighted average was calculated using the random effects model by the statistical software application, OpenMeta[Analyst].Citation4 The random effect model was preferred over the fixed effects model, as it accounts for heterogeneity between the different studies, primarily geographical and genetic differences.
Results
Article selection
After eliminating articles, from the 650 identified articles (), which did not contain the words ‘hydroxyurea’ and ‘thalassemia’ in their titles, only 85 articles remained for further screening. Of the 85 articles, 31 articles did not meet the inclusion criteria provided in the methodology section, leaving 54 articles. After applying the exclusion criteria () outlined in the methodology section, only 13 articles remained for quantitative analysis ().
Table 1. Number of articles included in meta-analysis
Table 2. Excluded articles
Table 3. Included articles with extracted information
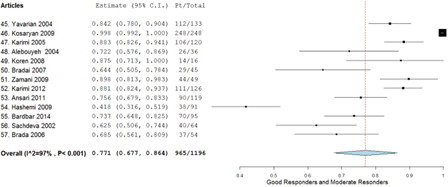
A total of 13 studies met all the inclusion and exclusion criteria, providing a total of 1196 patients. When the tabulated information of all the studies was combined, 572 patients were good responders, 393 patients were moderate responders, and 231 were poor responders (). The weighted average using the random effect model (P < 0.001) was determined to be 0.493 (95% confidence interval (CI), 0.387–0.599) for good responders (), 0.270 (95% CI, 0.166–0.375) for moderate responders (), and 0.229 (95% CI, 0.136–0.323) for poor responders (). Aggregation of the good and moderate responders generates a fourth category called ‘responders’. A total of 965 patients were aggregated into this category, and the weighted average using the random effect model (P < 0.001) was calculated to be 0.771 (95% CI, 0.677–0.864) ().
Table 4. Weighted averages using random effects model (P < 0.001)
Discussion
This meta-analysis was conducted in order to draw conclusions from existing studies on the efficacy of hydroxyurea therapy in reducing the number of transfusions needed in thalassemia patients. The results of each of the studies were divided as good, moderate, and poor responders depending upon the degree of decrease in blood transfusion requirements. Data on the levels of hemoglobin before and after hydroxyurea administration were not extracted, since pre-treatment hemoglobin levels were artificially elevated due to blood transfusions. The meta-analysis showed that 49.3% of the cumulative patient population were ‘good responders’. This is consistent with the study published in the American Society of Hematology's journal titled, ‘Hydroxyurea can eliminate transfusion requirements in children with severe B-thalassemia.’Citation5 This study followed seven children with transfusion-dependent B-thalassemia major in whom the need for blood transfusions was completely abolished after treatment with hydroxyurea. Another case study published in the New England Journal of Medicine titled, ‘Successful use of hydroxyurea in B-thalassemia major’Citation6 followed a 20-year-old male in whom blood transfusions were no longer effective due to the complications. After a year of therapy with hydroxyurea, the patient no longer required blood transfusions. Although both of these studies demonstrate the effects of hydroxyurea, their sample sizes were too small to make any generalizations.
The meta-analysis also showed that 27.0% of the study population were ‘moderate responders’. Although not eliminated, their need for transfusions decreased significantly, avoiding many of the side effects caused by their transfusion dependence. Many of the studies also mentioned decline in some of the other common complications of thalassemia. This illustrates the potential of hydroxyurea therapy in reducing thalassemia-associated complications and reducing the side effects of frequent blood transfusions.
The smallest proportion of the study population, only 22.9%, comprised of ‘poor responders’. A study published in the Journal of Pediatric Hematology/Oncology titled, ‘Efficacy of hydroxyurea in providing transfusion independence in b-thalassemia’Citation7 had similar results, where the smallest proportion of their study population, 20%, was of poor responders.
A fourth category, ‘responders’ was derived by combining the ‘good responders’ and ‘moderate responders’ and included 77.1% of the study population. This category shows the likelihood of a transfusion-dependent beta-thalassemia patient of showing substantial improvement after starting hydroxyurea therapy. It would be sensible for patients belonging to this group to continue with their hydroxyurea therapy.
A number of limitations of this study can be highlighted. Firstly, none of the studies included in this meta-analysis were double-blinded placebo-controlled trials. Up until the publishing of this article, no such study has been performed. Although this limits the ability of this study to draw definitive conclusions, it does help in suggesting the possible benefits of initiating hydroxyurea therapy on a trial basis. It is also hoped that this will help the initiation of double-blinded placebo-controlled studies in the future. The possible side effects of hydroxyurea on patients were not addressed in the meta-analysis. Some patients included in the studies experienced side effects to such an extent, they had to discontinue the drug; however, these cases were minimal.
Most of the included articles described their transfusion protocol. Generally, patients were transfused when hemoglobin levels were measured below 7 g/dl. When patients’ hemoglobin levels measured between 7 and 9 g/dl, additional clinical and laboratory criteria were considered before making the decision to transfuse. This threshold was lowered to 6 g/dl in certain regions experiencing shortages of blood products. Most authors considered the patient ‘transfusion independent’ if they were able to maintain their hemoglobin level above 9 g/dl. None of the articles specifically mentioned that the pre-treatment and post-treatment transfusion protocol was kept consistent. If the transfusion protocol was changed before or after treatment, this may adversely influence the results of study to show either an elevated or reduced effect of the hydroxyurea treatment. Since a change in transfusion protocol was not mentioned in any of the included articles, this meta-analysis assumed that they were kept consistent.
More studies need to be performed to help understand why some patients do not respond to hydroxyurea. Current attention has been focused on specific mutations, which may be more responsive to hydroxyurea treatment than others. Isolating the responsive mutations will help better identify the patients who will benefit from this therapy, and will help develop a better understanding of the underlying mechanism of hydroxyurea.
The duration of the included studies ranged from 4 to 156 months. In responding patients, effects of hydroxyurea were generally seen within 6 months. After years of treatment, some patients experienced transient dips in hemoglobin levels below 9 g/dl, a few being associated with flu-like symptoms. After a temporary period of increased transfusions, all patients were able to return to hydroxyurea treatment and maintain their hemoglobin level as they were before. This highlights the importance of continued monitoring of patients undergoing hydroxyurea therapy. Of the included studies, none of the authors mentioned the development of tolerance to hydroxyurea, even after years of ongoing treatment.
Although not included in this meta-analysis, many of the studies reviewed did mention significant increase in quality of life for patients showing response to hydroxyurea. These included more energy, better growth, and endurance.
Conclusion
This study suggests that there is a possible benefit to the use of hydroxyurea in patients with beta-thalassemia. Overall, the side effects of hydroxyurea are minimal, and the possible advantages are tremendous. Therefore, in certain clinical scenarios, it is understandable if a physician would like to start a trial therapy of hydroxyurea to patients with transfusion-dependent thalassemia, in particular, those who are unable to continue blood transfusions. Even if patients do not become completely transfusion independent, it may decrease the frequency of transfusions enough to avoid some of the transfusion-related complications.
In order to decisively conclude that hydroxyurea is effective in patients with transfusion-dependent beta-thalassemia, a comprehensive placebo-controlled double-blinded study should be performed.
Acknowledgements
The authors would like to thank all the authors of the works studied for their dedication to helping patients with thalassemia.
Disclaimer statements
Contributors KB and RK have contributed to this manuscript through research, gathering data, analyzing data, formulating research question, and writing up the manuscript.
Funding None.
Conflicts of interest None.
Ethics approval None.
References
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283(15):2008–12.
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Weels GA, Shea B, O'Connel D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available from: http://ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed June 2014).
- OpenMeta[Analyst] 2012. Available from: http://www.cebm.brown.edu/open_meta (accessed June 2014).
- Bradai M, Abad MT, Pissard S, Lamraoui F, Skopinski L, de Montalembert M. Hydroxyurea can eliminate transfusion requirements in children with severe B-thalassemia. Blood 2003;102(4):1529–30.
- Arruda VR, Lima CS, Saad ST, Costa FF. Successful use of hydroxyurea in beta-thalassemia major. N Engl J Med. 1997;336(13):964.
- Ansari SH, Shamsi TS, Siddiqui FJ, Irfan M, Perveen K, Farzana T, et al. Efficacy of hydroxyurea (HU) in reduction of pack red cell (PRC) transfusion requirement among children having beta-thalassemia major: Karachi HU trial (KHUT). J Pediatr Hematol Oncol. 2007;29(11):743–6.
- Ehsani MA, Hedayati-Asl A, Bagheri A, Zeinali S, Rashidi A. Hydroxyurea-induced hematological response in transfusion-dependent beta-thalassemia intermedia: case series and review of literature. Pediatr Hematol Oncol. 2009;26(8):560–5.
- Karimi M. Hydroxyurea in the management of thalassemia intermedia. Hemoglobin 2009;33(1):S177–82.
- de Paula EV, Lima CS, Arruda VR, Alberto FL, Saad ST, Costa FF. Long-term hydroxyurea therapy in beta-thalassaemia patients. Eur J Haematol. 2003;70(3):151–5.
- Taher A, Sheikh-Taha M. Hydroxyurea use in Lebanese patients with beta-thalassemia intermedia. J Pediatr Hematol Oncol. 2006;28(2):107.
- Kattamis A. Treatment of thalassemia with hydroxyurea: an indispensable alternative therapy. J Pediatr Hematol Oncol. 2007;29(11):729–30.
- Bohara VV, Ray S, Chakrabarti P, Ray SS, Nath UK, Chaudhuri U. Optimizing the dose of hydroxyurea therapy for patients with β-thalassemia intermedia (Hb E-β-thalassemia): a single center study from Eastern India. Hemoglobin 2014;38(1):44–8.
- Dixit A, Chatterjee TC, Mishra P, Choudhry DR, Mahapatra M, Tyagi S, et al. Hydroxyurea in thalassemia intermedia – a promising therapy. Ann Hematol. 2005;84(7):441–6.
- Italia KY, Jijina FJ, Merchant R, Panjwani S, Nadkarni AH, Sawant PM, et al. Response to hydroxyurea in beta thalassemia major and intermedia: experience in western India. Clin Chim Acta 2009;407(1–2):10–5.
- de Franceschi L, Rouyer-Fessard P, Alper SL, Jouault H, Brugnara C, Beuzard Y. Combination therapy of erythropoietin, hydroxyurea, and clotrimazole in a beta thalassemic mouse: a model for human therapy. Blood 1996;87(3):1188–95.
- Hoppe C, Vichinsky E, Lewis B, Foote D, Styles L. Hydroxyurea and sodium phenylbutyrate therapy in thalassemia intermedia. Am J Hematol. 1999;62(4):221–7.
- Karimi M, Mohammadi F, Behmanesh F, Samani SM, Borzouee M, Amoozgar H, et al. Effect of combination therapy of hydroxyurea with l-carnitine and magnesium chloride on hematologic parameters and cardiac function of patients with beta-thalassemia intermedia. Eur J Haematol. 2010;84(1):52–8.
- Dover GJ. Hemoglobin switching protocols in thalassemia. Experience with sodium phenylbutyrate and hydroxyurea. Ann N Y Acad Sci. 1998;850:80–6.
- Elalfy MS, Adly AA, Ismail EA, Elhenawy YI, Elghamry IR. Therapeutic superiority and safety of combined hydroxyurea with recombinant human erythropoietin over hydroxyurea in young β-thalassemia intermedia patients. Eur J Haematol. 2013;91(6):522–33.
- Mokhtar G, Tantawy AG, Abdel Moneim A, Waheed M. Thalassemia intermedia: an Egyptian experience. Haematologica 2009;94(2):241 abs. 0592.
- Loukopoulos D, Voskaridou E, Kalotychou V, Schina M, Loutradi A, Theodoropoulos I. Reduction of the clinical severity of sickle cell/beta-thalassemia with hydroxyurea: the experience of a single center in Greece. Blood Cells Mol Dis. 2000;26(5):453–66.
- Koren A, Segal-Kupershmit D, Zalman L, Levin C, Abu Hana M, Palmor H, et al. Effect of hydroxyurea in sickle cell anemia: a clinical trial in children and teenagers with severe sickle cell anemia and sickle cell beta-thalassemia. Pediatr Hematol Oncol. 1999;16(3):221–32.
- Rigano P, Manfré L, La Galla R, Renda D, Renda MC, Calabrese A, et al. Clinical and hematological response to hydroxyurea in a patient with Hb Lepore/beta-thalassemia. Hemoglobin 1997;21(3):219–26.
- Loukopoulos D, Voskaridou E, Stamoulakatou A, Papassotiriou Y, Kalotychou V, Loutradi A, et al. Hydroxyurea therapy in thalassemia. Ann N Y Acad Sci. 1998;850:120–8.
- Atweh GF, Loukopoulos D. Pharmacological induction of fetal hemoglobin in sickle cell disease and beta-thalassemia. Semin Hematol. 2001;38(4):367–73.
- Rigano P, Rodgers GP, Renda D, Renda MC, Aquino A, Maggio A. Clinical and hematological responses to hydroxyurea in Sicilian patients with Hb S/beta-thalassemia. Hemoglobin 2001;25(1):9–17.
- Panigrahi I, Dixit A, Arora S, Kabra M, Mahapatra M, Choudhry VP, et al. Do alpha deletions influence hydroxyurea response in thalassemia intermedia? Hematology 2005;10(1):61–3.
- Voskaridou E, Kalotychou V, Loukopoulos D. Clinical and laboratory effects of long-term administration of hydroxyurea to patients with sickle-cell/beta-thalassaemia. Br J Haematol. 1995;89(3):479–84.
- Italia KY, Jijina FF, Merchant R, Panjwani S, Nadkarni AH, Sawant PM, et al. Effect of hydroxyurea on the transfusion requirements in patients with severe HbE-beta-thalassaemia: a genotypic and phenotypic study. J Clin Pathol. 2010;63(2):147–50.
- Singer ST, Kuypers FA, Olivieri NF, Weatherall DJ, Mignacca R, Coates TD, et al. Single and combination drug therapy for fetal hemoglobin augmentation in hemoglobin E-beta 0-thalassemia: considerations for treatment. Ann N Y Acad Sci. 2005;1054:250–6.
- Fathallah H, Taher A, Bazarbachi A, Atweh GF. Differences in response to fetal hemoglobin induction therapy in beta-thalassemia and sickle cell disease. Blood Cells Mol Dis. 2009;43(1):58–62.
- Maggio A, Rigano P, Renda D. Treatment with hydroxyurea in sickle cell/β-thalassemia; a long-term experience. Am Soc Hematol Blood (ASH Annual Meeting Abstracts) 2004;104: Abstract 1678.
- Saxon BR, Rees D, Olivieri NF. Regression of extramedullary haemopoiesis and augmentation of fetal haemoglobin concentration during hydroxyurea therapy in beta thalassaemia. Br J Haematol. 1998;101(3):416–9.
- Karimi M, Cohan N, Pishdad P. Hydroxyurea as a first-line treatment of extramedullary hematopoiesis in patients with beta thalassemia: four case reports. Hematology 2015;20(1):53–7.
- Kohli-Kumar M, Marandi H, Keller MA, Guertin K, Hvizdala E. Use of hydroxyurea and recombinant erythropoietin in management of homozygous beta thalassemia. J Pediatr Hematol Oncol. 2002;24(9):777–8.
- Cario H, Wegener M, Debatin KM, Kohne E. Treatment with hydroxyurea in thalassemia intermedia with paravertebral pseudotumors of extramedullary hematopoiesis. Ann Hematol. 2002;81(8):478–82.
- Cianciulli P, Caravita T, Sorrentino F, Sergiacomi L, Massa A, Amadori S. Hydroxyurea therapy in paraparesis and cauda equina syndrome due to extramedullary haematopoiesis in thalassaemia: improvement of clinical and haematological parameters. Eur J Haematol. 2000;64:426–9.
- Zeng YT, Huang SZ, Ren ZR, Lu ZH, Zeng FY, Schechter AN, et al. Hydroxyurea therapy in beta-thalassaemia intermedia: improvement in haematological parameters due to enhanced beta-globin synthesis. Br J Haematol. 1995;90(3):557–63.
- Chik KW, Lee V, Shing MMK, Li CK. Hydroxyurea treatment in beta-thalassemia intermedia. HK J Pediatric. 2006;11:20–1.
- Rashid S. Transfusion therapy as a sole treatment option in a thalassemia patient with acute paraplegia – a case report and review of literature. Pak J Neurol Sci. 2012;7(3):15–20.
- Choudhry VP, Lal A, Pati HP, Arya LS. Hematological responses to hydroxyurea therapy in multitransfused thalassemic children. Indian J Pediatr. 1997;64(3):395–8.
- Hajfath A, Khavaran K, Kariman N, Zakeri H, Nikfarjam S, Valaei N. Effects of hydroxyurea on hematologic parameters of patients with thalassemia intermedia. Pejouhandeh 2004;86(36):373–8.
- Yavarian M, Fayazi N, Rostamani N, Shamsaei M, Karimi M. Efficacy and side effects of hydroxyurea in patients with thalassemia intermedia. Med J Hormozgan Univ. 2007;11(2):109–14.
- Yavarian M, Karimi M, Bakker E, Harteveld CL, Giordano PC. Response to hydroxyurea treatment in Iranian transfusion-dependent beta-thalassemia patients. Haematologica 2004;89(10):1172–78.
- Kosaryan M, Vahidshahi K, Karami H, Ehteshami S. Effect of hydroxyurea on thalassemia major and thalassemia intermedia in Iranian patients. Pak J Med Sci. 2009;25(1):74–8.
- Karimi M, Darzi H, Yavarian M. Hematologic and clinical responses of thalassemia intermedia patients to hydroxyurea during 6 year of therapy in Iran. J Pediatr Hematol Oncol. 2005;27(7):380–5.
- Alebouyeh M, Moussavi F, Haddad-Deylami H, Vossough P. Hydroxyurea in the treatment of major beta-thalassemia and importance of genetic screening. Ann Hematol. 2004;83(7):430–3.
- Koren A, Levin C, Dgany O, Kransnov T, Elhasid R, Zalman L, et al. Response to hydroxyurea therapy in beta-thalassemia. Am J Hematol. 2008;83(5):366–70.
- Bradai M, Pissard S, Abad MT, Dechartres A, Ribeil JA, Landais P, et al. Decreased transfusion needs associated with hydroxyurea therapy in Algerian patients with thalassemia major or intermedia. Transfusion 2007;47(10):1830–36.
- Zamani F, Shakeri R, Eslami SM, Razavi SM, Basi A. Hydroxyurea therapy in 49 patients with major beta-thalassemia. Arch Iran Med. 2009;12(3):295–7.
- Karimi M, Haghpanah S, Farhadi A, Yavarian M. Genotype-phenotype relationship of patients with β-thalassemia taking hydroxyurea: a 13-year experience in Iran. Int J Hematol. 2012;95(1):51–6.
- Ansari SH, Shamsi TS, Ashraf M, Perveen K, Farzana T, Bohray M, et al. Efficacy of hydroxyurea in providing transfusion independence in β-thalassemia. J Pediatr Hematol Oncol. 2011;33(5):339–43.
- Hashemi A, Abrishamkar M, Jenabzade AR, Eslami Z. Hydroxyurea can reduce or eliminate transfusion requirements in children with major and intermediate thalassemia. Iran J Blood Cancer 2009;1(4):147–50.
- Bordbar MR, Silavizadeh S, Haghpanah S, Kamfiroozi R, Bardestani M, Karimi M. Hydroxyurea treatment in transfusion-dependent β-thalassemia patients. Iran Red Crescent Med J. 2014;16(6):e18028.
- Sachdeva A, Khanna K, Verma C, Kaul K, Dinesh A, Subash C. Hydroxyurea and termination of transfusion therapy in transfusion dependent thalassemics. The 44th American Society of Hematology Annual Meeting, 2002, Detroit, USA.
- Bradai M, Pissard S, Abad MT, Dechartes A, Ribeil JA, Landais P, et al. Evolution of transfusion requirement in Algerian thalassemic major (TM) and intermediate (TI) patients treated with hydroxyurea (HU). Am Soc Hematol Blood 2006;108:1588.