Abstract
Objectives
Hematopoietic stem cell transplantation (HSCT) from a matched sibling donor (MSD) is the preferred initial treatment for children with severe aplastic anemia (SAA). Unfortunately, only about 30% of patients have a suitable human leukocyte antigen-matched sibling.
Methods
We have analyzed the outcome of 42 patients who received HSCT (22 MSD and 20 alternative donors (AD)) for SAA at the seven major pediatric HSCT centers in Mexico between 2001 and 2013.
Results
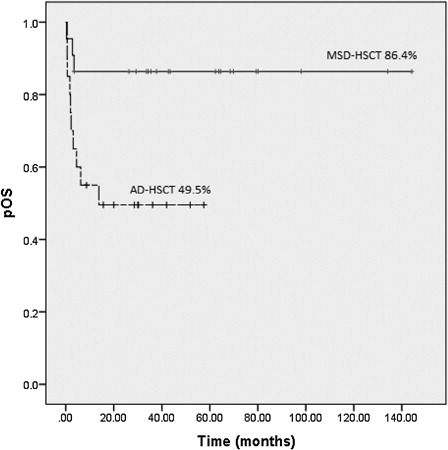
With a median follow-up of 30 months (range, 0.4–144), the 5-year overall survival in children transplanted from MSD was 86.4 + 7.3 vs. 49.5 + 11% for children after AD-HSCT (P = 0.013). The cumulative incidence of treatment-related mortality (TRM) was in the MSD-HSCT 9.1 + 3.9% vs. 47.6 + 9.1% in the AD-HSCT context (P = 0.007). Infectious complications contributed to death (91%) of most patients who received AD-HSCT.
Discussion
Even when the results of patients given MSD-HSCT are adequate, there is still much room for improvement particularly in children allografted with AD and in the supportive care. The development of an economicwise designed prospective project with MSD or matched unrelated donor HSCTs as a first line of treatment of children with SAA as a unified national trial could address these issues.
Introduction
Acquired severe aplastic anemia (SAA) is a rare, life-threatening disorder characterized by pancytopenia and bone marrow (BM) aplasia or hypoplasia.Citation1 Hematopoietic stem cell transplantation (HSCT) from a matched sibling donor (MSD) is the preferred initial treatment for children with SAA, with long-term survival rates reaching 80–95%.Citation2 Unfortunately, only about 30% of patients have a suitable human leukocyte antigen (HLA)-matched sibling.Citation3Alternative treatment for patients without an MSD is immunosuppressive therapy (IST). Survival rates after IST in children with SAA have been reported to be as high as 80%.Citation4–Citation6 Allogeneic HSCT using alternative donors (AD) is indicated when patients fail to respond to one or more courses of IST and for patients who experienced disease recurrence after IST.Citation7 The outcome of AD transplantations has been less encouraging, but recent data of patients undergoing AD-HSCT have significantly improved, especially for those who received MUD (matched unrelated donor) HSCT.Citation8–Citation14 We report the results of 42 children with SAA who underwent allogeneic (allo)-HSCT at the seven major pediatric HSCT centers in Mexico, and compare treatment outcomes between MSD vs. AD transplants.
Patients and methods
Patients
Data on 42 patients with SAA under the age of 18 years who underwent their first HSCT during 2001–2013 time period were analyzed retrospectively. Patients with congenital BM failure syndrome, such as Fanconi anemia, were excluded from this analysis. Patients' characteristics are listed in . Median times from diagnosis to transplantation were 12 months for all patients (range, 1–70 months), 6 months for MSD recipients, and 15 months for AD recipients.
Table 1. Patient characteristics
Donors' characteristics, stem cell source, and dose
Among the 42 donors, there were 22 MSD and 20 AD (2 MUD, 15 mismatched unrelated donors (MMUD), 1 mismatched related donor (MMRD) and 2 haploidentical). Of 22 patients transplanted from MSD, 14 received hematopoietic stem cells (HSC) from peripheral blood (peripheral blood stem cell (PBSC)), and 8 received BM. The source of HSC in AD was umbilical cord blood (UCB) in 17 cases and PBSC in 3 cases. Recipients of HSC from MSD received a median of 4.2 × 106 CD34+ cells/kg (range 1.1–9), while those transplanted from AD obtained a median of 0.36 × 106 CD34+ cells/kg (range 0.25–15.5).
Conditioning regimens
The type of conditioning regimens varied among participating institutions. The majority of MSD-HSCTs, 19 (86%) patients, was conditioning with a reduced-intensity regimen. Ten received cyclophosphamide (Cy) and fludarabine, seven received Cy and antithymocyte globulin (ATG), one patient received fludarabine and Cy + ATG, and one patient received only Cy. Three patients among the MSD-HSCTs received myeloablative regimens, two received busulfan and Cy + ATG, and one patient received BuCy. For AD-HSCTs, 14 (75%) patients were conditioned with a reduced-intensity regimen. Ten received Cy + ATG, one received Cy + fludarabine, two patients received fludarabine and Cy + ATG, and one patient received only Cy. Three patients among the AD-HSCTs that received a myeloablative regimen were conditioned with busulfan and Cy + ATG, two patients received BuCy, and one patient received fludarabine, melphalan, and Cy + ATG.
GVHD prophylaxis
Cyclosporin (CyA) (with serum trough levels 150–250 lg/L) plus methotrexate (MTX) pulses (days +1, +3, +6, and +11) were mainly used for gene-versus-host disease (GVHD) prophylaxis in both MSD- and AD-HSCTs. Alemtuzumab and CyA were used in one patient with haploidentical SCT, and mycophenolate mofetil and MTX were used in three other patients of AD-HSCTs group (one MMRD, one MUD, and one MMUD).
Chimerism studies
Fluorescence in situ hybridization probes for sex chromosomes were used for gender-mismatched donor/recipients. Otherwise, donor/recipient chimerism was evaluated by polymerase chain reaction amplification of specific polymorphic DNA sequences.
Infectious prophylaxis
All patients received fungal prophylaxis during the peritransplantation period, as well as acyclovir and all patients received Pneumocystis jirovecii pneumonia prophylaxis after engraftment. At some centers, bacterial prophylaxis was used prior to engraftment. When needed, patients received leukocyte-depleted ± irradiated blood products. Oral decontamination, mouth care, and a low microbial diet were also indicated. Monitoring of bacterial, viral, and fungal infections was carried out according to the institutional standard of care.
Statistical methods
Median, range, and proportions were used to summarize descriptive data, as appropriate. In the evaluation of engraftment (neutrophil count ≥ 0.5 × 109/L for 3 consecutive days and platelet count ≥ 20 × 109/L for 7 days, unsupported), patients who died before day +21 without engraftment were not considered evaluable. Cumulative incidence (CI) estimates were calculated for the probabilities of acute as well as chronic GVHD, and treatment-related mortality (TRM). Patients who failed to engraft were excluded from the analyses of acute and chronic GVHD. Patients who died before day +100 were excluded from analyses of chronic GVHD.
Survival time was calculated from date of transplantation to date of death or date of final patient follow-up. Probability of overall survival (OS) was estimated according to the Kaplan–Meier method. Comparisons between survival curves were performed by log-rank test. Multivariate analysis was performed using the Cox regression model. Statistical significance was defined as P < 0.05. Statistical calculations were performed using the SPSS software version 20.0 for Windows operating system (SPSS, Cary, NC, USA).
Results
Engraftment and graft failure incidence
Thirty-eight of 42 transplants were evaluable for engraftment. Engraftment was achieved in 95.2% (20/21) of MSD-HSCT and in 53% (9/17) of AD-HSCT, evaluable for engraftment recipients (P = 0.005). Median duration of time to neutrophil and platelet engraftment after MSD-HSCT was 16 days (range 12–68) and 18 days (range 8–110), while for AD-HSCT it was 42 days (range 11–75) and 50 days (range 15–100), respectively (P = 0.008). Neutrophil engraftment was faster after peripheral blood stem cell transplantation PBSCT (median, day 15; range, 11–21), when compared with BM (day 23; 14– 68) or UCB-HSCTs (day 43; 24–75) (P = 0.005). Primary graft failure occurred only in one (4.8%) patient after MSD-HSCT and in nine (53%) after AD-HSCT, while there was secondary graft failure in three (17.6%) recipients of AD-HSCT, and in no recipients after MSD-HSCT (P < 0.001). Of note graft failure occurred in 78.6% (11/14) of the UCBT. Twenty-five of 38 (65.7%) evaluable transplant patients had 100% donor chimerism. Twenty of 21 (95.2%) MSD recipients and 5 of 17 (29.4%) AD recipients were 100% chimeric. One AD transplant recipient had mixed chimerism but died from a cause not related with SAA or TRM. Two patients had autologous recovery (AD recipients) and are now transfusion-independent with 100% recipient cells. Univariate analysis showed that HLA-match level was the only factor predicting engraftment for AD-HSCT (P = 0.047). Other factors analyzed that showed no significance were as follows: number of previous transfusions; time from diagnosis to transplant; previous IST; conditioning regimen; donor/recipient gender combination; year of transplantation; source of stem cells; and number of stem cells.
Graft-versus-host disease
The incidence of severe acute GVHD (aGVHD) (grade III or IV), and extensive cGVHD for the entire cohort was 9.7 ± 3.2%, and 10.5 ± 2.9%, respectively. There was no significant difference in the incidence of severe aGVHD (P = 0.434) and cGVHD (P = 0.362) among MSD and AD transplants. Due to the sample size, the source of HSC was not a significant factor; however, it might be important to mention that in the two cases with severe aGVHD, the HSC received were from PB. To date, the two cases of cGVHD have been controllable.
OS and factors influencing survival
With a median follow-up of 30 months (range 0.4–144), 5-year OS in children transplanted from MSD was 86.4 ± 7.3 vs. 49.5 ± 11% for children after AD-HSCT (P = 0.013) (). There were three (13.6%) deaths after MSD-HSCT, one patient died due to infection (sepsis); one died due to graft rejection, and in the third one, the cause was unknown, as there were a lack of treatment compliance and a loss of follow-up.
After AD-HSCT, 10 (50%) children died, graft rejection and infection were the causes of death in 8 patients:
| • | neafter haploidentical HSCT, and seven after UCB-HSCTs (six MMUD and one MUD), aGVHD and infection were the causes in one patient after haploidentical HSCT, and one patient with an MMRD-HSCT, died 14 months after transplant in an accident. To date, there have been no reports of second malignancies. The CI of TRM was in the MSD-HSCT 9.1 ± 3.9 vs. 47.6 ± 9.1% in the AD-HSCT (P = 0.007). Univariate analysis showed a significant difference in OS among patients with or without graft failure (P < 0.001). In multivariate analysis, MSD and engraftment were independent predictors of survival (P = 0.023 and P = 0.011, respectively). Other factors analyzed that showed no significance were as follows: number of previous transfusions; time from diagnosis to transplant; previous IST; conditioning regimen; donor/recipient gender combination; year of transplantation; source of stem cells; and number of stem cells. | ||||
Discussion
There is a lack of recent data of the SAA incidence in Mexican pediatric population. We estimated that, given the current pediatric population in Mexico and according to the historical data of aplastic anemia (AA),Citation15 there are approximately 150 new cases of AA each year in Mexico, and the majority of cases are receiving IST as initial therapy; however, there are no reports of the outcome with IST in these patients either. Even though, IST has achieved improvement in survival, but is associated with high rates of clonal evolution and relapse.Citation16–Citation19 On the other hand, the risk of graft failure after HSCT may be increased due to a higher number of unavoidable pretransplant transfusions in children who had first been treated ineffectively with IST and who then, time after qualified for AD-HSCT.Citation20,Citation21 Usually, the lack of financial support limits the use of filtered or irradiated blood products, before transplantation in developing countries. These methods are considered standard clinical practice in the treatment of newly diagnosed SAA patient, and probably contributed to the lower rejection risk in centers from developed countries.Citation22 Statistical analysis in this study showed that neither the number of units transfused prior to HSCT nor the time interval between diagnosis and HSCT to have impact on graft failure. However, it is important to note that most of the patients in both groups had received >10 transfusions (67.4%) and at least one IST (95.3%). This fact reflects the problems and time required to obtain funding for the HSCT, even for the MSD cases.
The results of our patients with MSD-HSCT are comparable with those informed in several publications.Citation8,Citation9,Citation20,Citation23–Citation26 It is worth to mention that in order to simplify and cut off the high cost of the procedure in 41% (9/22) of the patients, HSCT was performed on an outpatient basis, as per protocol at two of the centers, and only one of these patients died as a consequence of graft rejection. Outpatient HSCT cannot be offered indiscriminately to all patients; but could be a good option for asymptomatic, fully active patients with reliable caregivers. This approach has resulted in a substantial reduction in cost without serious complications, and has been a reasonable option in México. It has been considered that nonmanipulated BM must be used as the stem cell source for children with AA. Despite earlier engraftment with the use of PBSCs, an increased risk of chronic GVHD has been described by some, as well as survival advantage for BM in all age groups.Citation26 Nevertheless, in México, there is a trend to increase the use of PBSC in order to obtain earlier engraftment in our high-risk, overtransfused children with AA. In this study, no significant negative impact of the cell source was observed, and the development of acute and chronic GVHD was lower than expected at least in the setting of MSD transplants.
In our patients, the outcome is significantly less favorable than that reported by other centers in children who underwent AD-HSCT;Citation8–Citation10,Citation12–Citation14,Citation24 this is due to a significant difference in TRM between MSD- and AD-HSCT . Infectious complications were the sole cause of TRM in one patient transplanted from MSD, but contributed to most of the deaths (91%) of patients who received AD-HSCT. Graft failure with or without infection was the main cause of TRM in our entire cohort, accounting for 71.4% of the 14 deaths. Only one patient transplanted from AD died due to aGVHD.
However, data from registry studies also displayed dismal outcomes for umbilical cord HSCT,Citation27–Citation29 and AD-HSCT for SAA has promoted similar outcomes compared with MSD-HSCT only when fully HLA-matched or partially HLA-MUDs were used.Citation12
Outcome in recent years in the patients undergoing AD-HSCT has significantly improved, with similar results to those for MSD-HSCT, showing an excellent OS, ranging from 75 to 95%.Citation8–Citation10, Citation12–Citation14, Citation24 Thus, many authors have suggested that AD-HSCT, especially MUD-HSCT, could be considered a first-line treatment for pediatric patients with SAA for whom a molecularly MUD is available. Of note, one recent study based on a large number of patients with SAA concluded that single- or multiple allele mismatches restricted to HLA-C, DRB1, and DQB1 are acceptable in the unrelated donor transplant setting, which expands the opportunity to find a good donor option.Citation30 Unfortunately, for most patients without an MSD, the search of donor had to be limited to Mexico due to economic issue; this was also the reason why most of the AD-HSCTs were from UCB, as the donor registry for MUD is very limited in our country.
It is difficult to make a decision on the best way to treat an SAA patient who lacks an MSD in a country such as Mexico with upper middle-income level, but simultaneously great economic inequality and limited public health care resources. There is the outcome of our AD-HSCT (only two MUD cases) as a second-line of treatment, which highlighted our urgent need to improve the supportive care and experience in this kind of transplant. Furthermore, it is difficult to maintain long-term supportive care in developing countries, and earlier MUD-HSCT could reduce the risk of nonengraftment related to alloimmunization resulting from pretransplant transfusions, and therefore also reduce the time of greater risk of infections. As referred by Szpecht et al.,Citation20 the high rate of lethal infections after AD-HSCT could be related to extreme and long-lasting IST given as a first-line treatment, which is often complicated by severe infections, and then followed by an intense immunosuppressive conditioning regimen for transplantation.
In conclusion, although this study is retrospective, it has a low number of patients, and the AD-HSCT group is rather heterogeneous; it provides a good picture of our current situation and the areas to be improved upon, which could be similar in other developing countries. Our data encourage early HLA typing and donor search soon after diagnosis in children with SAA, as well as deep evaluation and improvement of the supportive care, along with an economicwise designed prospective project with MSD or MUD-HSCTs as a first line of treatment, such as a unified national trial.
Disclaimer statements
Contributors LRR designed the study, assembled the data, performed statistical analysis, and wrote the manuscript. DGA, GJRA, and OGR edited the manuscript. OGR was the principal investigator, providing support, and takes primary responsibility for the paper. All authors contributed to HSCT, acquisition of data, interpretation of data, review, and approval of the final manuscript.
Funding None.
Conflicts of interest The authors declare no conflict of interest.
Ethics approval Ethical approval was obtained from Institutional Ethics Committee.
References
- Young NS. Aplastic anaemia. Lancet 1995;346(8969):228–32.
- Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 2007;92(1):11–8.
- Storb R, Blume KG, O'Donnell MR, Chauncey T, Forman SJ, Deeg HJ, et al. Cyclophosphamide and antithymocyte globulin to condition patients with aplastic anemia for allogeneic marrow transplantations: the experience in four centers. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2001;7(1):39–44.
- Yoshida N, Yagasaki H, Hama A, Takahashi Y, Kosaka Y, Kobayashi R, et al. Predicting response to immunosuppressive therapy in childhood aplastic anemia. Haematologica 2011;96(5):771–4.
- Fuhrer M, Rampf U, Baumann I, Faldum A, Niemeyer C, Janka-Schaub G, et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood 2005;106(6):2102–04.
- Pongtanakul B, Das PK, Charpentier K, Dror Y. Outcome of children with aplastic anemia treated with immunosuppressive therapy. Pediatr Blood Cancer 2008;50(1):52–7.
- Korthof ET, Bekassy AN, Hussein AA. Management of acquired aplastic anemia in children. Bone Marrow Transplant. 2013;48(2):191–5.
- Chen J, Lee V, Luo CJ, Chiang AK, Hongeng S, Tan PL, et al. Allogeneic stem cell transplantation for children with acquired severe aplastic anaemia: a retrospective study by the Viva-Asia Blood and Marrow Transplantation Group. Br J Haematol. 2013;162(3):383–91.
- Kennedy-Nasser AA, Leung KS, Mahajan A, Weiss HL, Arce JA, Gottschalk S, et al. Comparable outcomes of matched-related and alternative donor stem cell transplantation for pediatric severe aplastic anemia. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2006;12(12):1277–84.
- Bacigalupo A, Socie G, Lanino E, Prete A, Locatelli F, Locasciulli A, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica 2010;95(6):976–82.
- Perez-Albuerne ED, Eapen M, Klein J, Gross TJ, Lipton JM, Baker KS, et al. Outcome of unrelated donor stem cell transplantation for children with severe aplastic anemia. Br J Haematol. 2008;141(2):216–23.
- Yagasaki H, Takahashi Y, Hama A, Kudo K, Nishio N, Muramatsu H, et al. Comparison of matched-sibling donor BMT and unrelated donor BMT in children and adolescent with acquired severe aplastic anemia. Bone Marrow Transplant. 2010;45(10):1508–13.
- Hsieh MY, Chiou TJ, Hung GY, Yen HJ. Outcomes of matched sibling and alternative donor stem cell transplantation for 26 children with severe aplastic anemia. Int J Hematol. 2010;91(1):54–60.
- Samarasinghe S, Steward C, Hiwarkar P, Saif MA, Hough R, Webb D, et al. Excellent outcome of matched unrelated donor transplantation in paediatric aplastic anaemia following failure with immunosuppressive therapy: a United Kingdom multicentre retrospective experience. Br J Haematol. 2012;157(3):339–46.
- Benitez-Aranda H, Velez-Ruelas MA, Diaz-Cardenas S, Sanchez-Valle E, Xolotl-Castillo M, Duenas-Gonzalez MT, et al. Incidence of aplastic anemia in a defined subpopulation from Mexico City. Hematology 2002;7(4):229–32.
- Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H, German Aplastic Anemia Study G. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood 2003;101(4):1236–42.
- Locasciulli A, Bruno B, Rambaldi A, Saracco P, Dufour C, Finelli C, et al. Treatment of severe aplastic anemia with antilymphocyte globulin, cyclosporine and two different granulocyte colony-stimulating factor regimens: a GITMO prospective randomized study. Haematologica 2004;89(9):1054–61.
- Locasciulli A, Arcese W, Locatelli F, Di Bona E, Bacigalupo A, Italian Aplastic Anaemia Study G. Treatment of aplastic anaemia with granulocyte-colony stimulating factor and risk of malignancy. Italian Aplastic Anaemia Study Group. Lancet 2001;357(9249):43–4.
- Kojima S, Ohara A, Tsuchida M, Kudoh T, Hanada R, Okimoto Y, et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood 2002;100(3):786–90.
- Szpecht D, Gorczynska E, Kalwak K, Owoc-Lempach J, Choma M, Styczynski J, et al. Matched sibling versus matched unrelated allogeneic hematopoietic stem cell transplantation in children with severe acquired aplastic anemia: experience of the polish pediatric group for hematopoietic stem cell transplantation. Arch Immunol Ther Exp. 2012;60(3):225–33.
- Srinivasan R, Takahashi Y, McCoy JP, Espinoza-Delgado I, Dorrance C, Igarashi T, et al. Overcoming graft rejection in heavily transfused and allo-immunised patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br J Haematol. 2006;133(3):305–14.
- Burroughs LM, Woolfrey AE, Storer BE, Deeg HJ, Flowers ME, Martin PJ, et al. Success of allogeneic marrow transplantation for children with severe aplastic anaemia. Br J Haematol. 2012;158(1):120–8.
- Kim H, Lee JH, Joo YD, Bae SH, Hyun MS, Lee JH, et al. A randomized comparison of cyclophosphamide vs. reduced dose cyclophosphamide plus fludarabine for allogeneic hematopoietic cell transplantation in patients with aplastic anemia and hypoplastic myelodysplastic syndrome. Ann Hematol. 2012;91(9):1459–69.
- Chung NG, Lee JW, Jang PS, Jeong DC, Cho B, Kim HK. Reduced dose cyclophosphamide, fludarabine and antithymocyte globulin for sibling and unrelated transplant of children with severe and very severe aplastic anemia. Pediatr Transplant. 2013;17(4):387–93.
- Kikuchi A, Yabe H, Kato K, Koh K, Inagaki J, Sasahara Y, et al. Long-term outcome of childhood aplastic anemia patients who underwent allogeneic hematopoietic SCT from an HLA-matched sibling donor in Japan. Bone Marrow Transplant. 2013;48(5):657–60.
- Bacigalupo A, Socie G, Schrezenmeier H, Tichelli A, Locasciulli A, Fuehrer M, et al. Bone marrow versus peripheral blood as the stem cell source for sibling transplants in acquired aplastic anemia: survival advantage for bone marrow in all age groups. Haematologica 2012;97(8):1142–8.
- Eapen M, Horowitz MM. Alternative donor transplantation for aplastic anemia. Hematol Am Soc Hematol Educ Program 2010;2010:43–6.
- MacMillan ML, Walters MC, Gluckman E. Transplant outcomes in bone marrow failure syndromes and hemoglobinopathies. Semin Hematol. 2010;47(1):37–45.
- Yoshimi A, Kojima S, Taniguchi S, Hara J, Matsui T, Takahashi Y, et al. Unrelated cord blood transplantation for severe aplastic anemia. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2008;14(9):1057–63.
- Yagasaki H, Kojima S, Yabe H, Kato K, Kigasawa H, Sakamaki H, et al. Acceptable HLA-mismatching in unrelated donor bone marrow transplantation for patients with acquired severe aplastic anemia. Blood 2011;118(11):3186–90.